Alternative models for studying Aspergilli Dr Peter Warn











- Slides: 49

Alternative models for studying Aspergilli Dr Peter Warn School of Translational Medicine University of Manchester peter. warn@manchester. ac. uk

First The Good News • This should be the only slide you need to take notes from. • This presentation will be available at http: //www. aspergillus. org. uk/ • Any SOPs referred to will be available through the same link • Additional SOPs will be available through the IAAM website http: //www. sacmm. org/iaam. html

Why do we need models of aspergillosis? Ø To provide a bridge between in vitro studies and clinical research – Models have been the bedrock of research under pinning many research areas üUnderstanding Innate and adaptive immunity üPathogenesis üVirulence üDrug discovery

Desirable attributes of animal models 1 ØMirror diseases seen in humans as closely as possible ØPredictive of clinical outcomes ØModels are standardized ØReproducible ØEasy to set-up and require little specialist equipment ØReasonable cost Chamilos et al. Lancet Infect Dis 2007; 7: 42 -55. Clemons & Stevens. Med Mycol 2005; 43: S 101 -10.

Desirable attributes of animal models 2 ØAmenable to studies including * Evaluation of therapeutics * Evaluation of host response * Evaluation of pathogen virulence factors * Assessment of in vivo gene expression Chamilos et al. Lancet Infect Dis 2007; 7: 42 -55. Clemons & Stevens. Med Mycol 2005; 43: S 101 -10.

Weaknesses of Animal Models ØWill never fully replicate human disease ØNo single model answers all questions ØMay not mimic all structural features e. g. the structure of mouse lung ØAdditional effort with drug studies to ‘humanize’ PK and metabolic effects ØAnimal models can be acute and expensive Chamilos et al. Lancet Infect Dis 2007; 7: 42 -55. Clemons & Stevens. Med Mycol 2005; 43: S 101 -10.

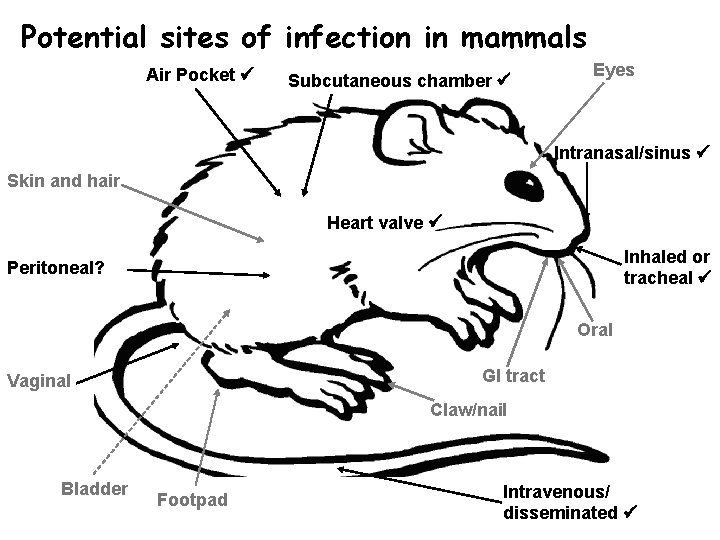
Potential sites of infection in mammals Air Pocket Subcutaneous chamber Eyes Intranasal/sinus Skin and hair Heart valve Inhaled or tracheal Peritoneal? Oral GI tract Vaginal Claw/nail Bladder Footpad Intravenous/ disseminated

Modulators of fungal infection – host factors • Age of animal – in general younger animals more susceptible • Genetic background inbred v outbred – only mice • Immune status Immunocompetent: Immunocompromised: neutropenic vs. non-neutropenic • Tissue damage • Sex - Hormone status • Site of infection - route of infection/ method of infection • Pre exposure to whole fungi- hyphae or spores – immune status • Sensitization with fungal allergens

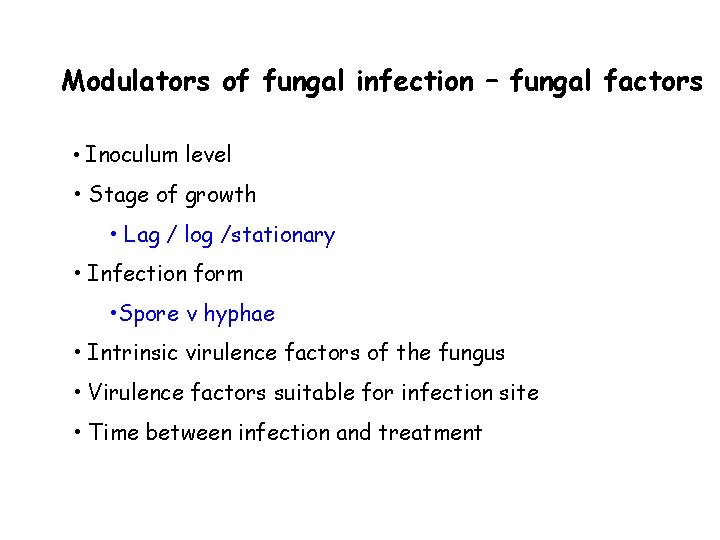
Modulators of fungal infection – fungal factors • Inoculum level • Stage of growth • Lag / log /stationary • Infection form • Spore v hyphae • Intrinsic virulence factors of the fungus • Virulence factors suitable for infection site • Time between infection and treatment

Housing and Husbandry Clean dedicated animal housing Day/Night light cycles Controlled temperature/humidity Room sterilization possible between models Waste disposal

Housing and Husbandry

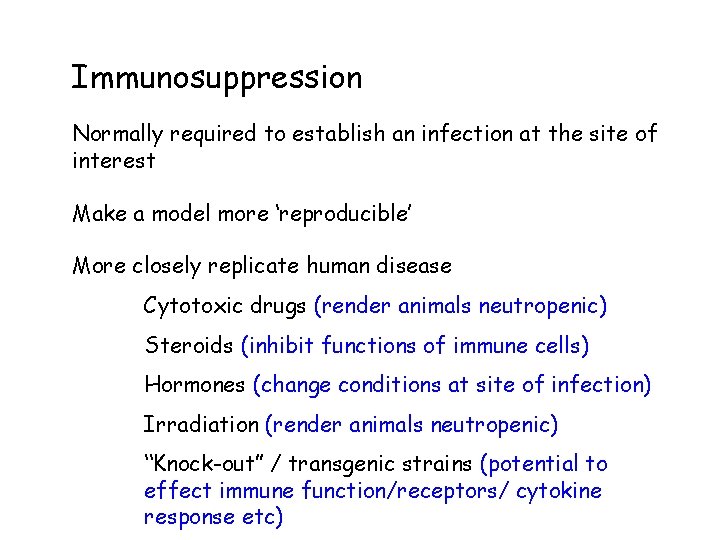
Immunosuppression Normally required to establish an infection at the site of interest Make a model more ‘reproducible’ More closely replicate human disease Cytotoxic drugs (render animals neutropenic) Steroids (inhibit functions of immune cells) Hormones (change conditions at site of infection) Irradiation (render animals neutropenic) “Knock-out” / transgenic strains (potential to effect immune function/receptors/ cytokine response etc)

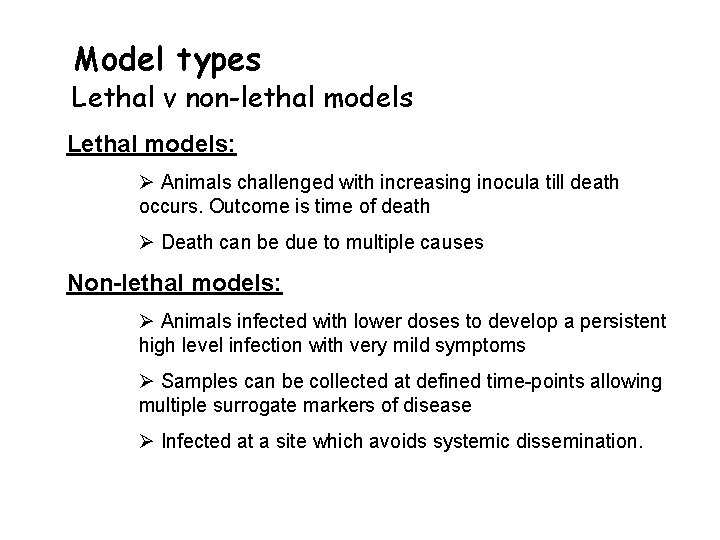
Model types Lethal v non-lethal models Lethal models: Ø Animals challenged with increasing inocula till death occurs. Outcome is time of death Ø Death can be due to multiple causes Non-lethal models: Ø Animals infected with lower doses to develop a persistent high level infection with very mild symptoms Ø Samples can be collected at defined time-points allowing multiple surrogate markers of disease Ø Infected at a site which avoids systemic dissemination.

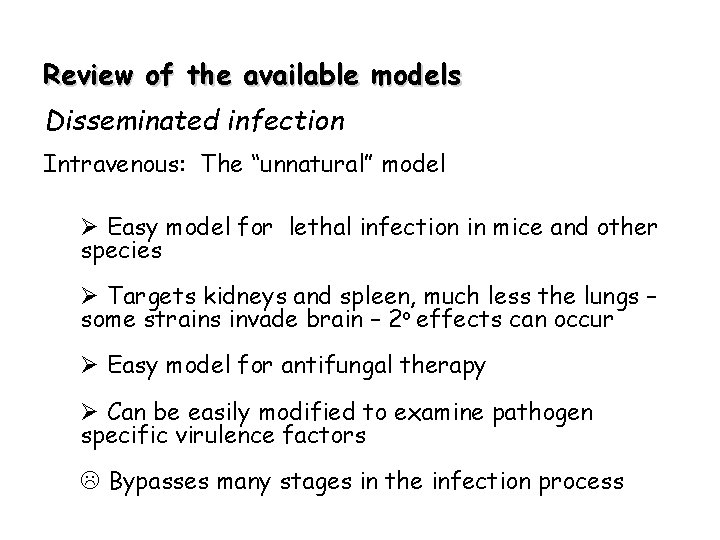
Review of the available models Disseminated infection Intravenous: The “unnatural” model Ø Easy model for lethal infection in mice and other species Ø Targets kidneys and spleen, much less the lungs – some strains invade brain – 2 o effects can occur Ø Easy model for antifungal therapy Ø Can be easily modified to examine pathogen specific virulence factors L Bypasses many stages in the infection process

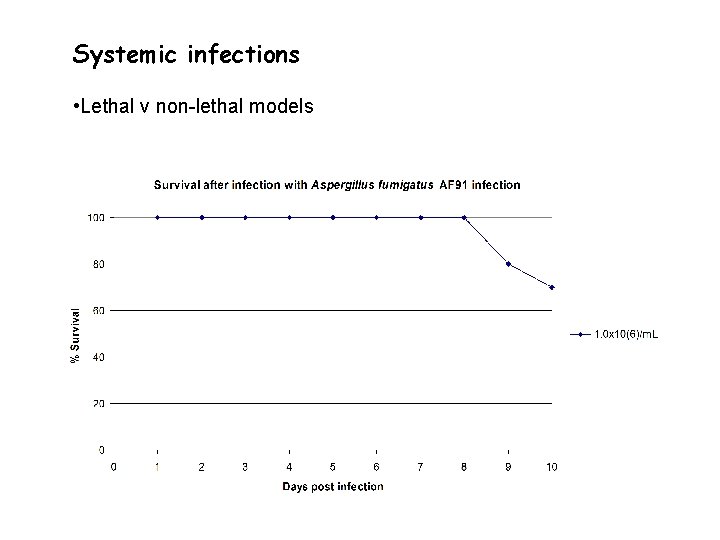
Systemic infections • Lethal v non-lethal models

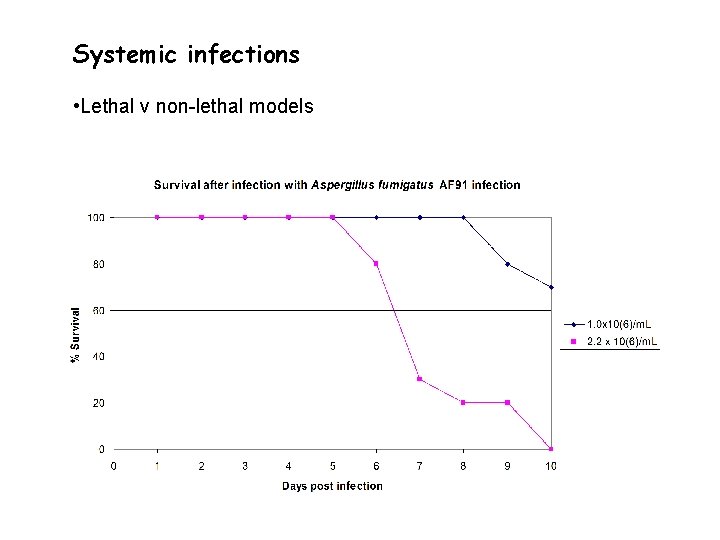
Systemic infections • Lethal v non-lethal models

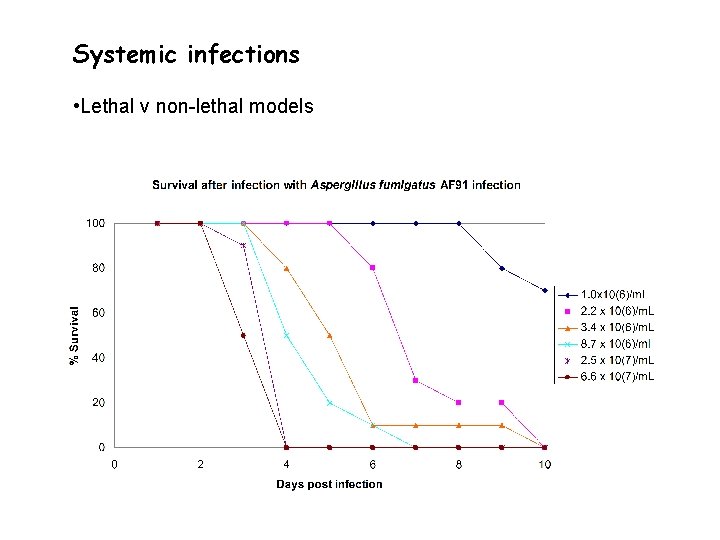
Systemic infections • Lethal v non-lethal models

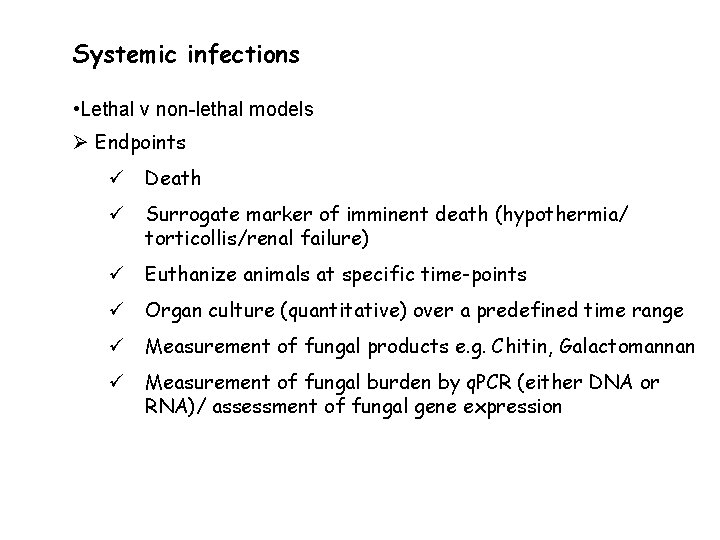
Systemic infections • Lethal v non-lethal models Ø Endpoints ü Death ü Surrogate marker of imminent death (hypothermia/ torticollis/renal failure) ü Euthanize animals at specific time-points ü Organ culture (quantitative) over a predefined time range ü Measurement of fungal products e. g. Chitin, Galactomannan ü Measurement of fungal burden by q. PCR (either DNA or RNA)/ assessment of fungal gene expression

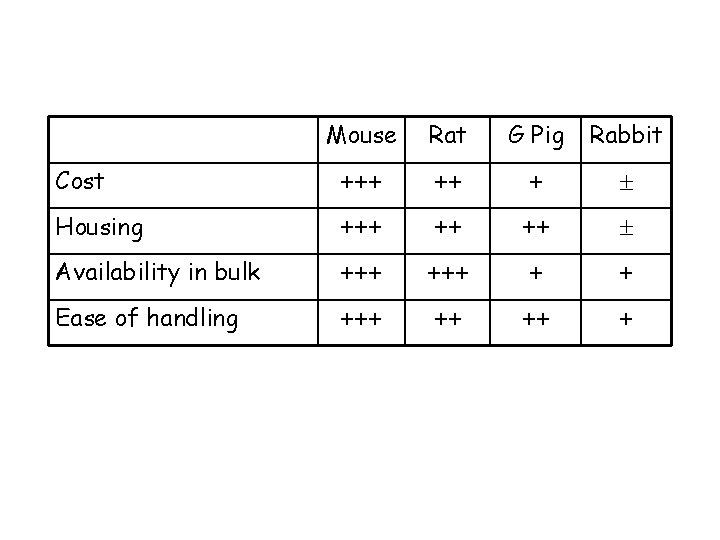
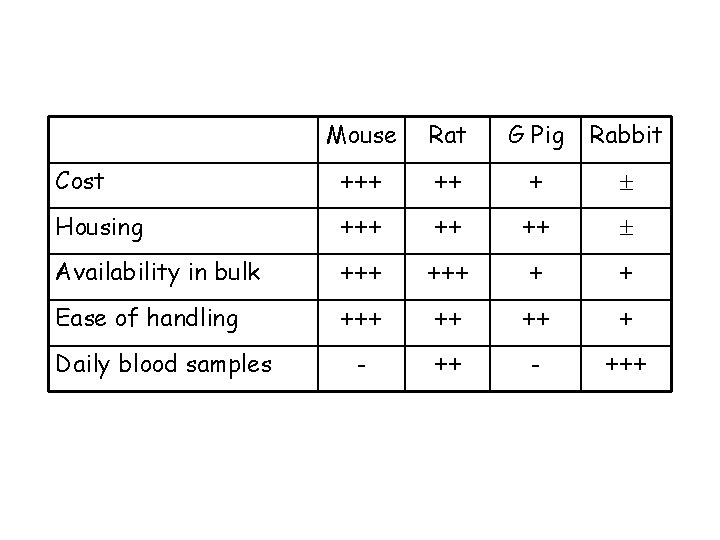
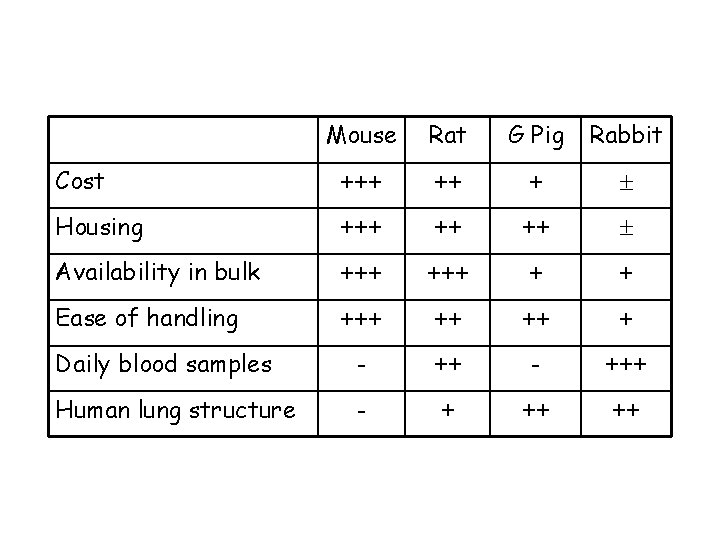
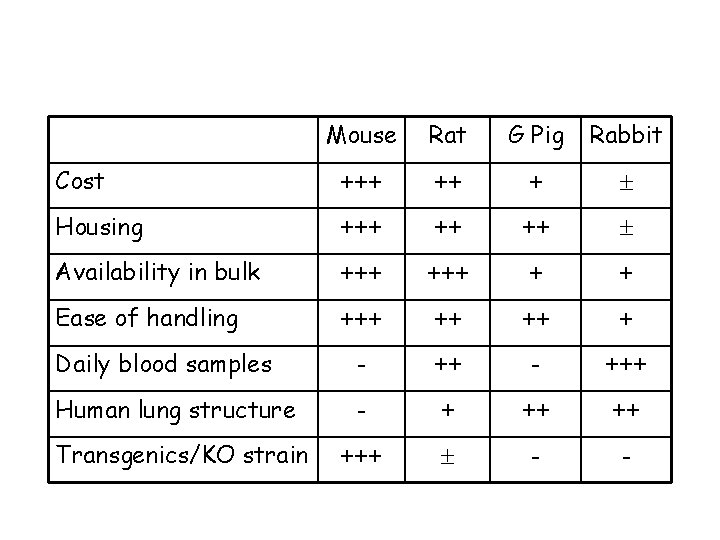
Review of the available models Mice versus other rodents Advantages: ØCan study disease in mice with specific host immune defects…potentially identifying the most critical ØCan study disease in large numbers of fairly uniform inbred animals … increasing reproducibility of results ØLess space for housing ØCost ØEase of handling Disadvantages: ØSerial sampling not usually possible ØLung remodelling/airway narrowing differs from larger animals ØDrugs are cleared from mice far more rapidly than in humans ØCourse of disease generally very acute, leading to death or recovery

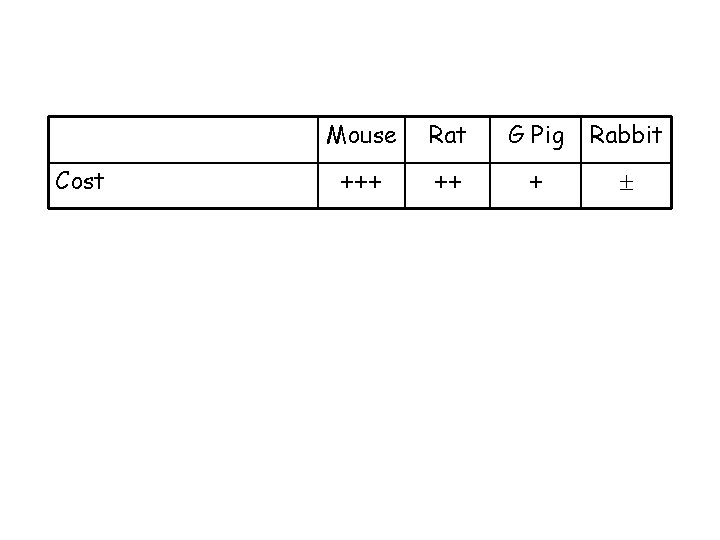
Cost Mouse Rat G Pig Rabbit +++ ++ +

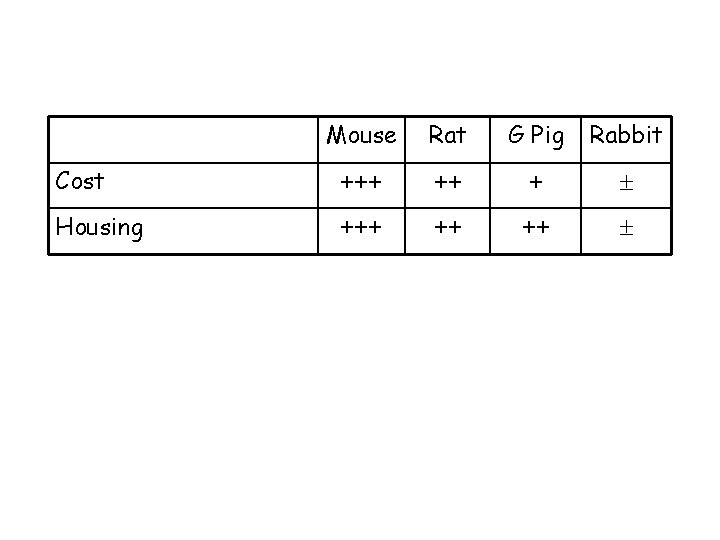
Mouse Rat G Pig Rabbit Cost +++ ++ + Housing +++ ++ ++

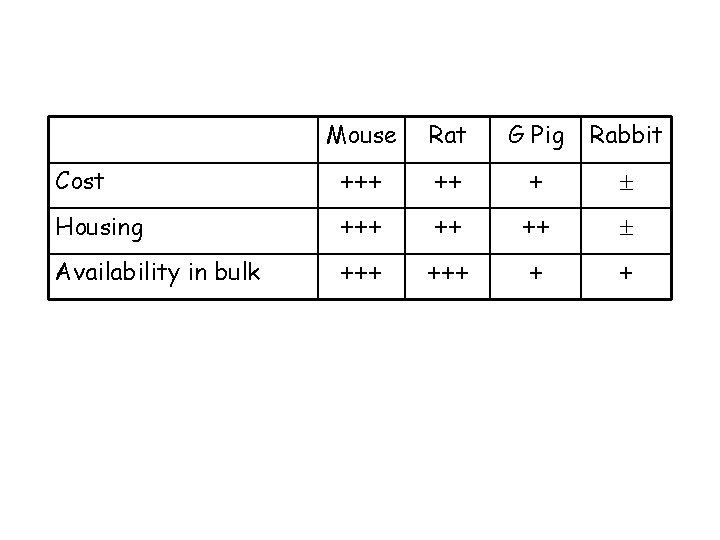
Mouse Rat G Pig Rabbit Cost +++ ++ + Housing +++ ++ ++ Availability in bulk +++ + +

Mouse Rat G Pig Rabbit Cost +++ ++ + Housing +++ ++ ++ Availability in bulk +++ + + Ease of handling +++ ++ ++ +

Mouse Rat G Pig Rabbit Cost +++ ++ + Housing +++ ++ ++ Availability in bulk +++ + + Ease of handling +++ ++ ++ + - +++ Daily blood samples

Mouse Rat G Pig Rabbit Cost +++ ++ + Housing +++ ++ ++ Availability in bulk +++ + + Ease of handling +++ ++ ++ + Daily blood samples - +++ Human lung structure - + ++ ++

Mouse Rat G Pig Rabbit Cost +++ ++ + Housing +++ ++ ++ Availability in bulk +++ + + Ease of handling +++ ++ ++ + Daily blood samples - +++ Human lung structure - + ++ ++ Transgenics/KO strain +++ - -

Models of localized infections a) Invasive Pulmonary aspergillosis Most models of IPA use infection by direct intranasal/intratracheal inoculation • Mice are anaesthetized and conidia suspension inhaled • Rats, Guinea pigs & Rabbits infected via tracheostomy/ intubation Advantages ü Relatively cost effective ü Little specialist equipment required ü Possible to infect large numbers from a single organism stock ü Possible to test multiple strains in a single model

Models of localized infections a) Invasive Pulmonary aspergillosis Most models of IPA use infection by direct intranasal/intratracheal inoculation • Mice are anaesthetized and conidia suspension inhaled • Rats, Guinea pigs & Rabbits infected via tracheostomy/ intubation Drawbacks L Enormouse-mouse variation - direct methods better L Inter-laboratory studies difficult L Distribution may not be equal between lobes L L Inoculum delivered in liquid – assumption that all of the inoculum delivered to lungs Some animals develop bacterial pneumonia L Animals develop disease in trachea or sinuses L Therapeutic studies difficult

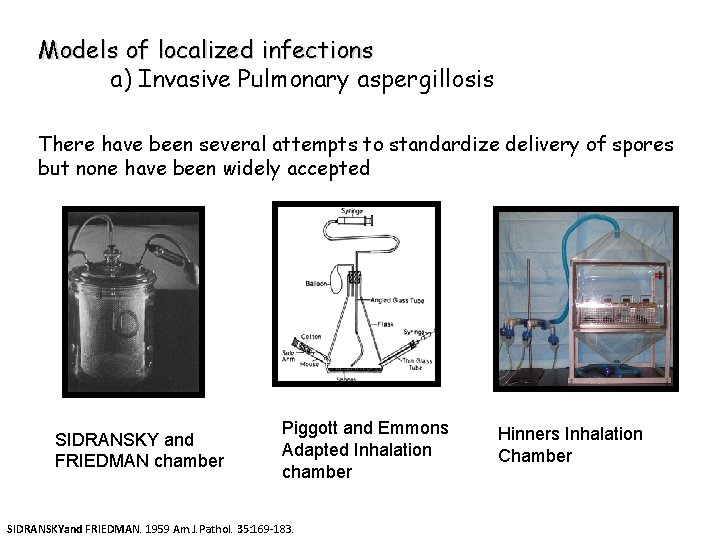
Models of localized infections a) Invasive Pulmonary aspergillosis There have been several attempts to standardize delivery of spores but none have been widely accepted SIDRANSKY and FRIEDMAN chamber Piggott and Emmons Adapted Inhalation chamber SIDRANSKYand FRIEDMAN. 1959 Am. J. Pathol. 35: 169 -183. Hinners Inhalation Chamber

• Development and standardization of aerosol challenge model of invasive pulmonary aspergillosis • Mouse, rat, guinea pig • Provide samples and resources to other investigators • Supported by NIH / NIAID • UTHSCSA / Harbor-UCLA / University of Manchester http: //www. sacmm. org/iaam. html

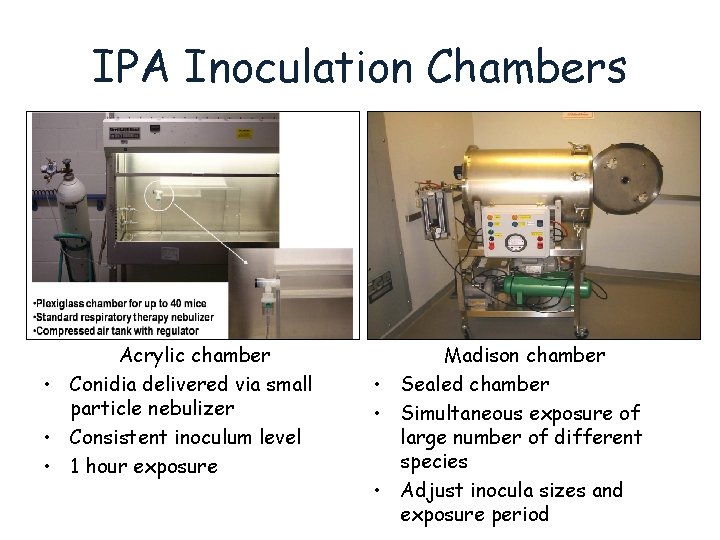
IPA Inoculation Chambers Acrylic chamber • Conidia delivered via small particle nebulizer • Consistent inoculum level • 1 hour exposure Madison chamber • Sealed chamber • Simultaneous exposure of large number of different species • Adjust inocula sizes and exposure period

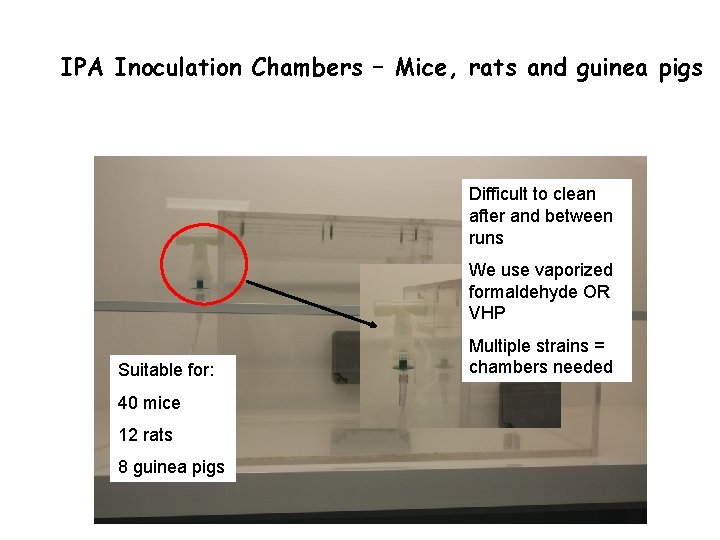
IPA Inoculation Chambers – Mice, rats and guinea pigs

IPA Inoculation Chambers – Mice, rats and guinea pigs Difficult to clean after and between runs We use vaporized formaldehyde OR VHP Suitable for: 40 mice 12 rats 8 guinea pigs Multiple strains = chambers needed

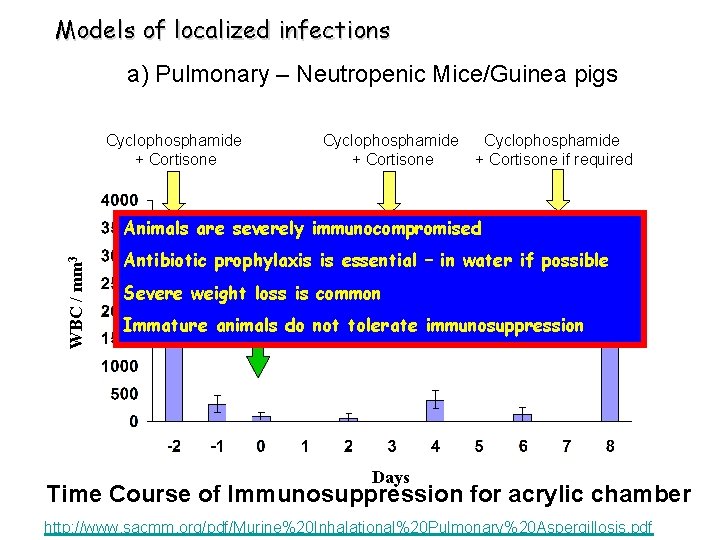
Models of localized infections a) Pulmonary – Neutropenic Mice/Guinea pigs Cyclophosphamide + Cortisone if required WBC / mm 3 Animals are severely immunocompromised Antibiotic prophylaxis is essential – in water if possible Infect Severe weight loss is common Immature animals do not tolerate immunosuppression Days Time Course of Immunosuppression for acrylic chamber http: //www. sacmm. org/pdf/Murine%20 Inhalational%20 Pulmonary%20 Aspergillosis. pdf

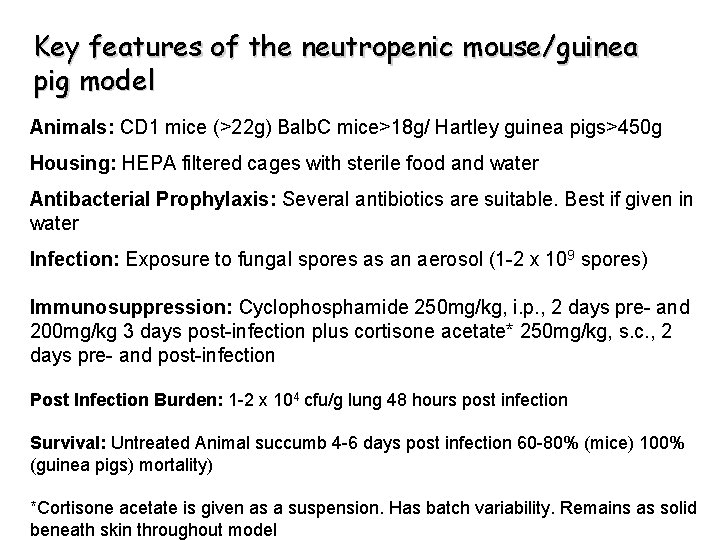
Key features of the neutropenic mouse/guinea pig model Animals: CD 1 mice (>22 g) Balb. C mice>18 g/ Hartley guinea pigs>450 g Housing: HEPA filtered cages with sterile food and water Antibacterial Prophylaxis: Several antibiotics are suitable. Best if given in water Infection: Exposure to fungal spores as an aerosol (1 -2 x 109 spores) Immunosuppression: Cyclophosphamide 250 mg/kg, i. p. , 2 days pre- and 200 mg/kg 3 days post-infection plus cortisone acetate* 250 mg/kg, s. c. , 2 days pre- and post-infection Post Infection Burden: 1 -2 x 104 cfu/g lung 48 hours post infection Survival: Untreated Animal succumb 4 -6 days post infection 60 -80% (mice) 100% (guinea pigs) mortality) *Cortisone acetate is given as a suspension. Has batch variability. Remains as solid beneath skin throughout model

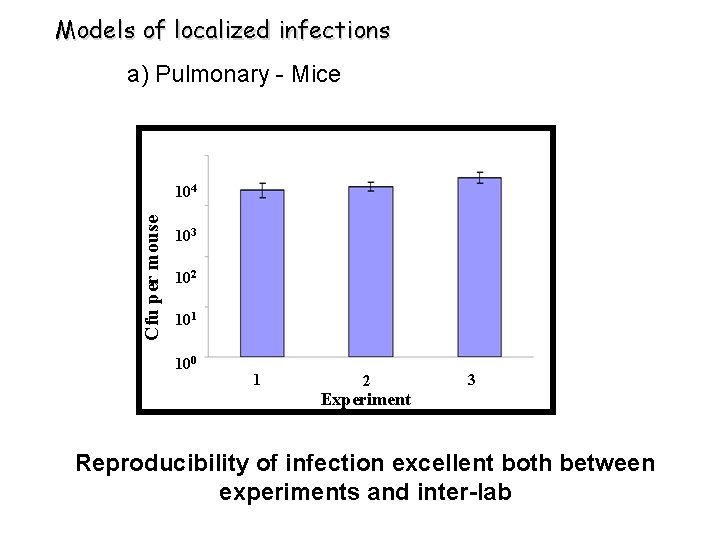
Models of localized infections a) Pulmonary - Mice Cfu per mouse 104 103 102 101 100 1 2 3 Experiment Reproducibility of infection excellent both between experiments and inter-lab

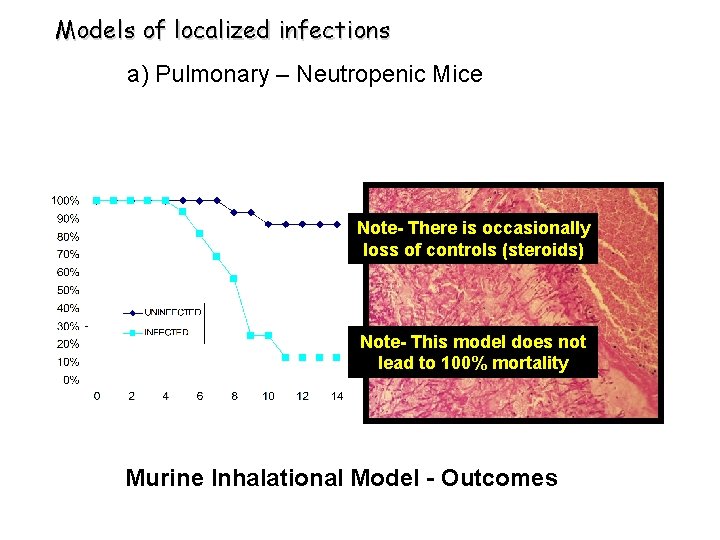
Models of localized infections a) Pulmonary – Neutropenic Mice Note- There is occasionally loss of controls (steroids) Note- This model does not lead to 100% mortality Murine Inhalational Model - Outcomes

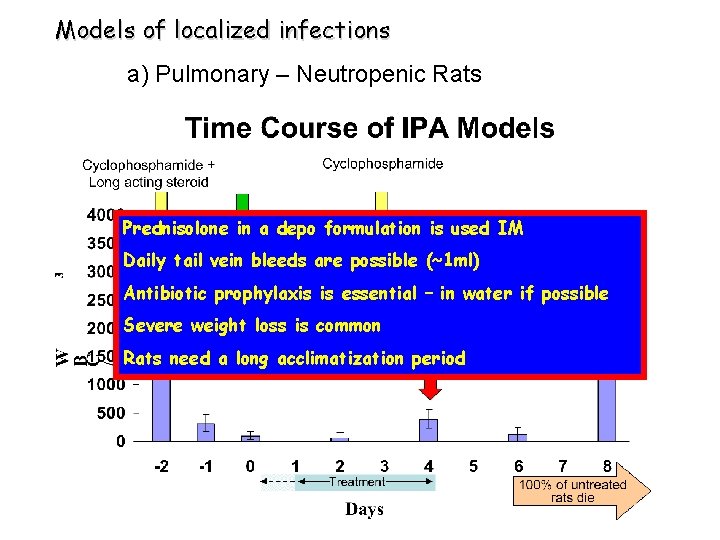
Models of localized infections a) Pulmonary – Neutropenic Rats Prednisolone in a depo formulation is used IM Daily tail vein bleeds are possible (~1 ml) Antibiotic prophylaxis is essential – in water if possible Severe weight loss is common Rats need a long acclimatization period

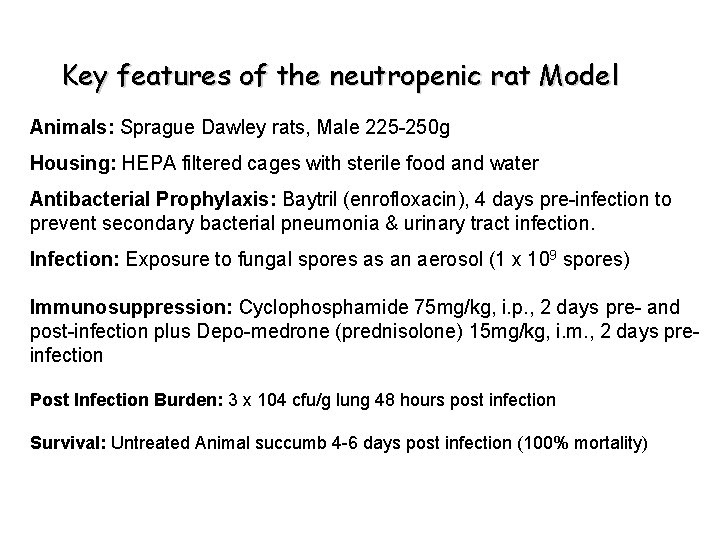
Key features of the neutropenic rat Model Animals: Sprague Dawley rats, Male 225 -250 g Housing: HEPA filtered cages with sterile food and water Antibacterial Prophylaxis: Baytril (enrofloxacin), 4 days pre-infection to prevent secondary bacterial pneumonia & urinary tract infection. Infection: Exposure to fungal spores as an aerosol (1 x 109 spores) Immunosuppression: Cyclophosphamide 75 mg/kg, i. p. , 2 days pre- and post-infection plus Depo-medrone (prednisolone) 15 mg/kg, i. m. , 2 days preinfection Post Infection Burden: 3 x 104 cfu/g lung 48 hours post infection Survival: Untreated Animal succumb 4 -6 days post infection (100% mortality)

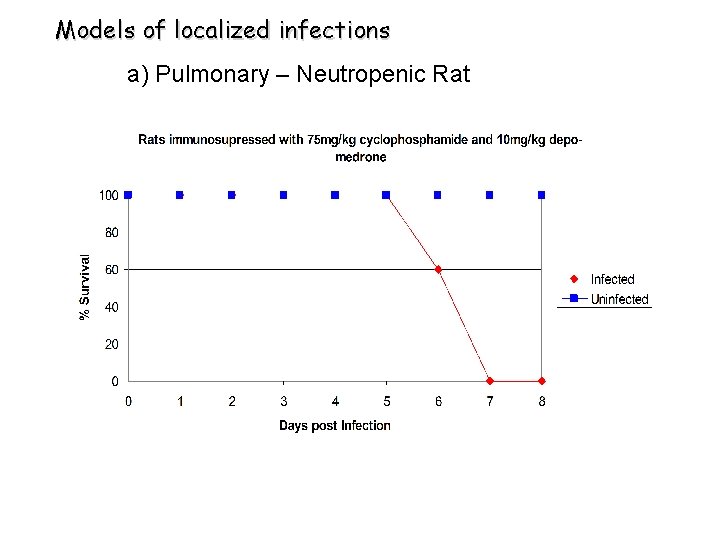
Models of localized infections a) Pulmonary – Neutropenic Rat

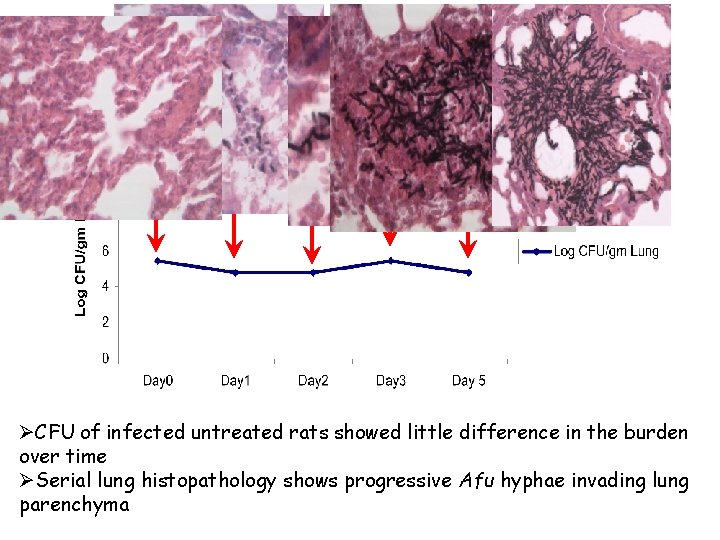
Aspergillus Burden Changes During Infection ØCFU of infected untreated rats showed little difference in the burden over time ØSerial lung histopathology shows progressive Afu hyphae invading lung parenchyma

Characteristic disease progression in rats Experimental endpoints: Major causes of death Weight loss >25% Laboured breathing Bloody nasal discharge Unable to reach food and water

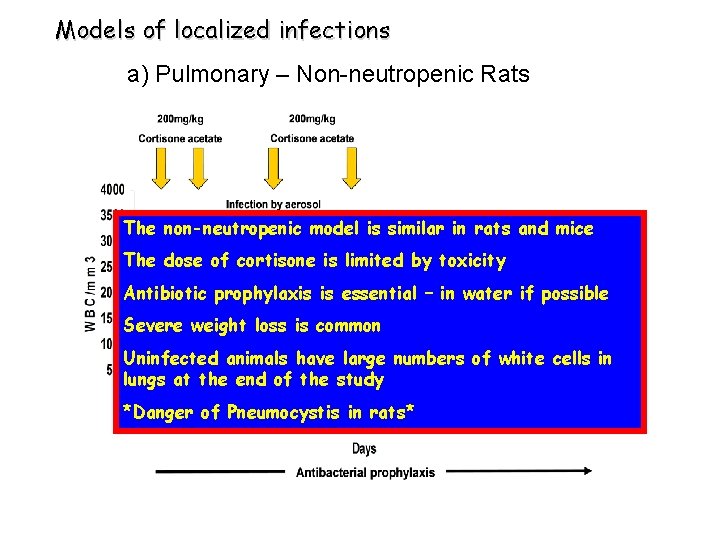
Models of localized infections a) Pulmonary – Non-neutropenic Rats The non-neutropenic model is similar in rats and mice The dose of cortisone is limited by toxicity Antibiotic prophylaxis is essential – in water if possible Severe weight loss is common Uninfected animals have large numbers of white cells in lungs at the end of the study *Danger of Pneumocystis in rats*

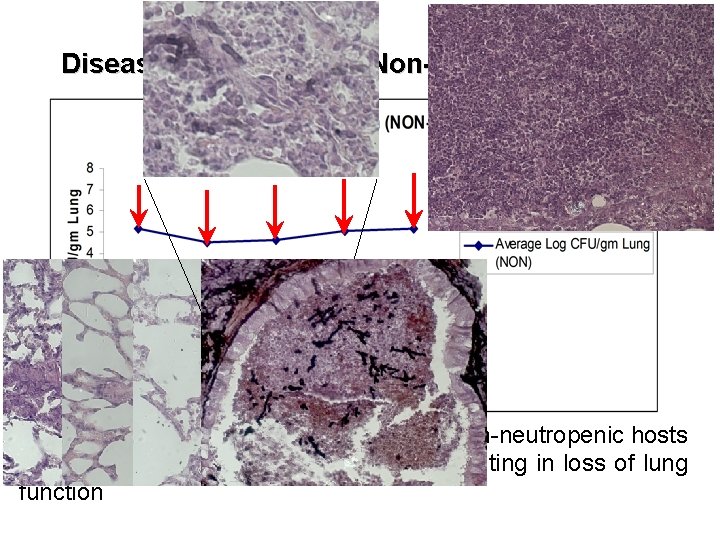
Disease Progression – Non-neutropenic rats The lung pathology following infection in non-neutropenic hosts is dominated by white cell recruitment resulting in loss of lung function

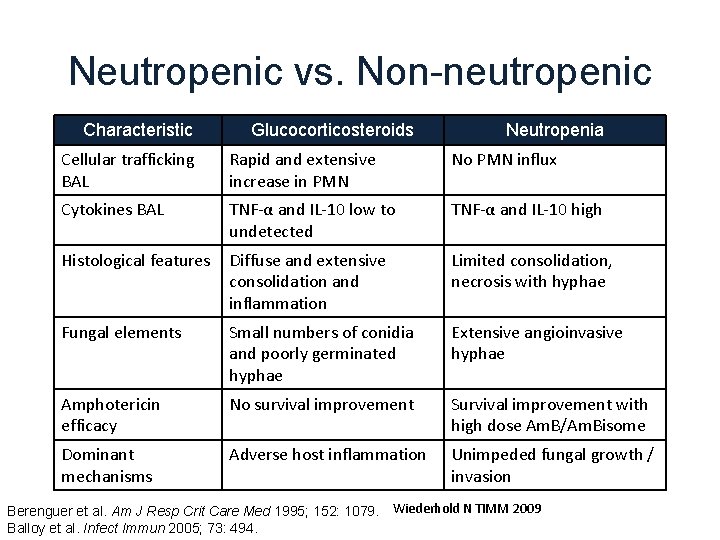
Neutropenic vs. Non-neutropenic Characteristic Glucocorticosteroids Neutropenia Cellular trafficking BAL Rapid and extensive increase in PMN No PMN influx Cytokines BAL TNF-α and IL-10 low to undetected TNF-α and IL-10 high Histological features Diffuse and extensive consolidation and inflammation Limited consolidation, necrosis with hyphae Fungal elements Small numbers of conidia and poorly germinated hyphae Extensive angioinvasive hyphae Amphotericin efficacy No survival improvement Survival improvement with high dose Am. B/Am. Bisome Dominant mechanisms Adverse host inflammation Unimpeded fungal growth / invasion Berenguer et al. Am J Resp Crit Care Med 1995; 152: 1079. Wiederhold N TIMM 2009 Balloy et al. Infect Immun 2005; 73: 494.

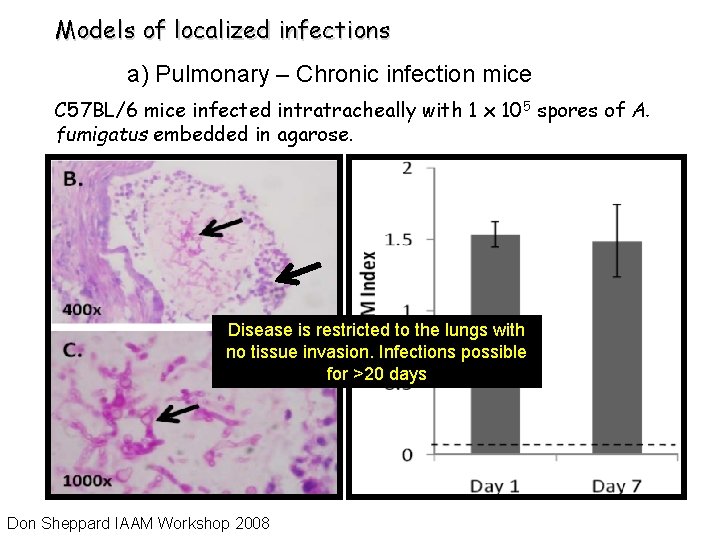
Models of localized infections a) Pulmonary – Chronic infection mice C 57 BL/6 mice infected intratracheally with 1 x 105 spores of A. fumigatus embedded in agarose. Disease is restricted to the lungs with no tissue invasion. Infections possible for >20 days Don Sheppard IAAM Workshop 2008

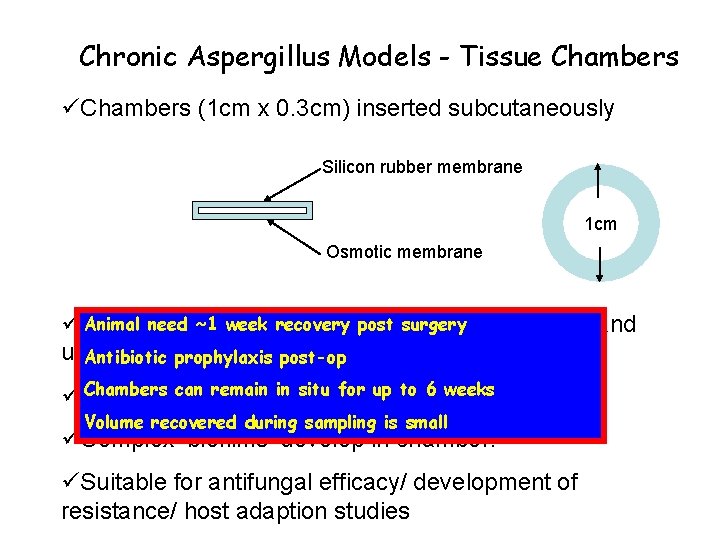
Chronic Aspergillus Models - Tissue Chambers üChambers (1 cm x 0. 3 cm) inserted subcutaneously Silicon rubber membrane 1 cm Osmotic membrane Animal need ~1 recoveryfrom post cellular surgery responses and üAspergillus is week separated unable to invade beyond the chamber. Antibiotic prophylaxis post-op Chambers can remain in situ forsilicon up to 6 membrane weeks üSampling possible though Volume recovered during sampling is small üComplex ‘biofilms’ develop in chamber. üSuitable for antifungal efficacy/ development of resistance/ host adaption studies

No time to discuss other models • Rabbits – great for drug and imaging studies • Transgenic/knockout mouse models – fantastic for understanding disease mechanisms • Non-mammalian hosts • Sinus models • Allergy/Asthma

Acknowledgements • • Andrew Sharp Raghdaa Shrief Jayesh Majithiya Joanne Slater David Denning University of Manchester IAAM Contract team Fungal Research Trust