Alternative Clinical Trial Designs for Research on Hypertensive









- Slides: 14

Alternative Clinical Trial Designs for Research on Hypertensive Disorders of Pregnancy in Low Resource Settings Dr Shivaprasad S Goudar MD, MHPE Professor of Physiology & Research Coordinator, Women's and Children's Health Research Unit, KLE University's Jawaharlal Nehru Medical College, Belgaum, India

1. Community Level Interventions for Preeclampsia - CLIP Trial Cluster Randomized Controlled Trial 2. CRADLE 3 (Community Blood Pressure Measurement in Rural Africa and Asia: the Detection of Underlying Pre-eclampsia and Shock) Stepped-Wedge Randomised Control Trial

Community Level Intervention for Pre-eclampsia CLIP INDIA TRIAL

Community Level Interventions for Preeclampsia-Cluster Randomized Controlled Trial (CLIP Trial) • Objectives – To reduce pre-eclampsia related maternal and perinatal mortality and major morbidity by 20% – To reduce pre-eclampsia related adverse maternal and perinatal events using cost-effective interventions appropriate for low resource settings.

CLIP Trial- Study Design

Field implementation of Android app for guiding management of Pre-Eclampsia/Eclampsia

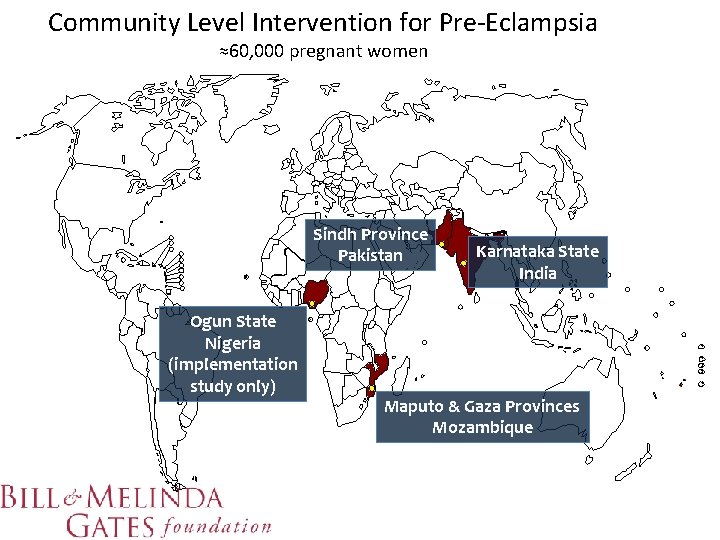
Community Level Intervention for Pre-Eclampsia ≈60, 000 pregnant women Sindh Province Pakistan Ogun State Nigeria (implementation study only) Karnataka State India Maputo & Gaza Provinces Mozambique

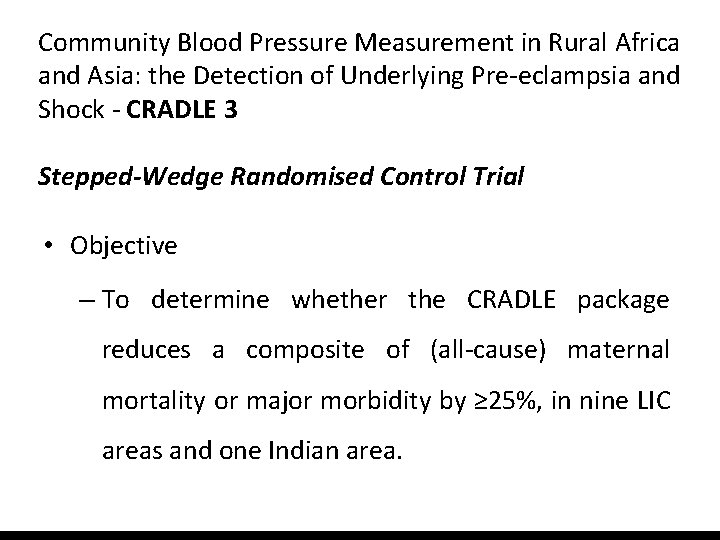
Community Blood Pressure Measurement in Rural Africa and Asia: the Detection of Underlying Pre-eclampsia and Shock - CRADLE 3 Stepped-Wedge Randomised Control Trial • Objective – To determine whether the CRADLE package reduces a composite of (all-cause) maternal mortality or major morbidity by ≥ 25%, in nine LIC areas and one Indian area.

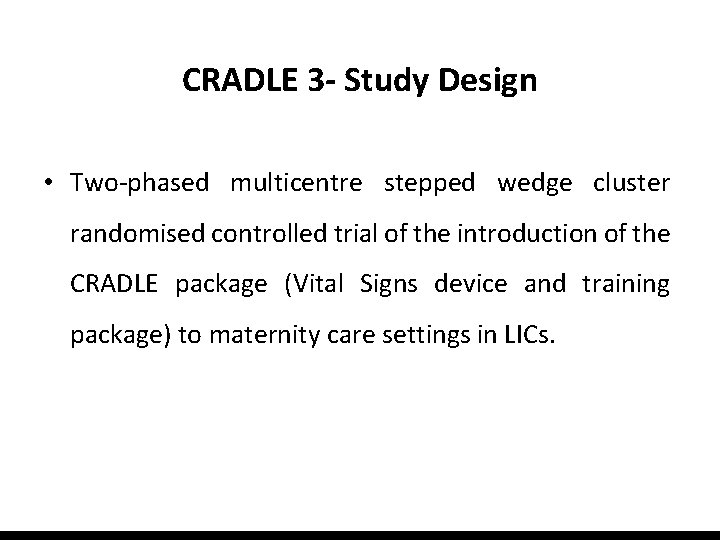
CRADLE 3 - Study Design • Two-phased multicentre stepped wedge cluster randomised controlled trial of the introduction of the CRADLE package (Vital Signs device and training package) to maternity care settings in LICs.

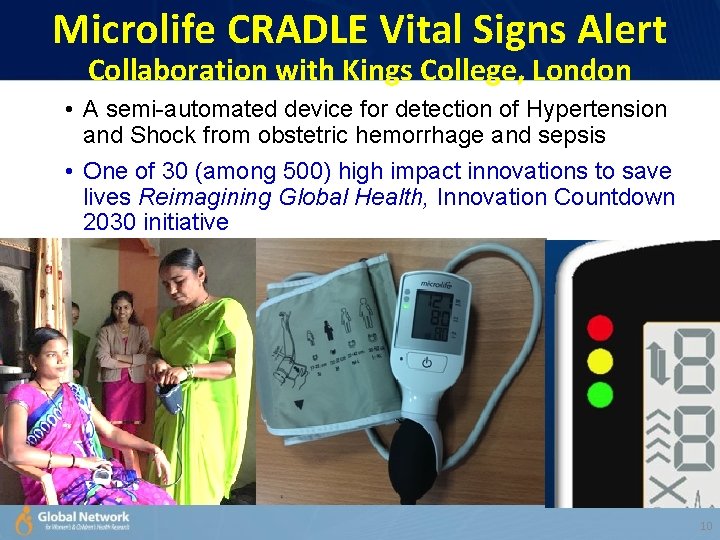
Microlife CRADLE Vital Signs Alert Collaboration with Kings College, London • A semi-automated device for detection of Hypertension and Shock from obstetric hemorrhage and sepsis • One of 30 (among 500) high impact innovations to save lives Reimagining Global Health, Innovation Countdown 2030 initiative 10

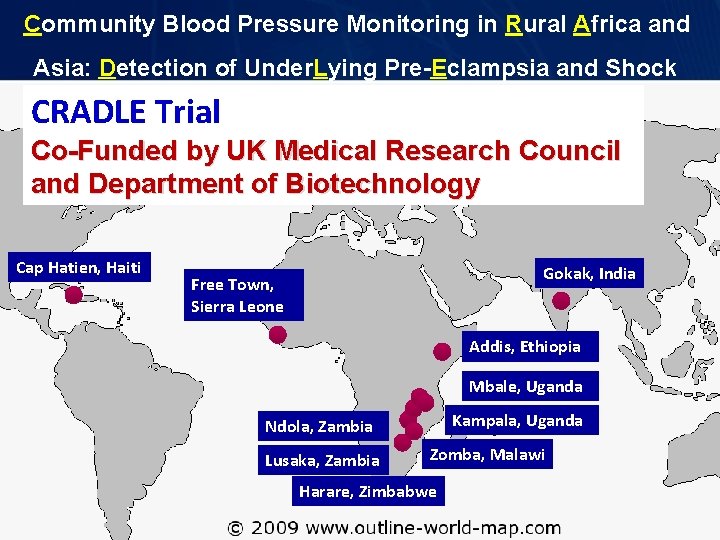
Community Blood Pressure Monitoring in Rural Africa and Asia: Detection of Under. Lying Pre-Eclampsia and Shock CRADLE Trial Co-Funded by UK Medical Research Council and Department of Biotechnology Cap Hatien, Haiti Gokak, India Free Town, Sierra Leone Addis, Ethiopia Mbale, Uganda Kampala, Uganda Ndola, Zambia Lusaka, Zambia Zomba, Malawi Harare, Zimbabwe Page 11

Ethical Issues: Cluster RCTs • Individual autonomy vs community consent • Task shifting of clinical work to Community Health Workers • Contamination in control clusters and common referral facilities • Implementing “standard of care” in control clusters per ministry guidelines, an intervention in itself?

Ethical Issues: Stepped-Wedged RCTs • Collection of aggregate outcome data at facility level; waiver of individual consent • Avoids contamination, the pre-randomization period serves as its own control • Because of randomization at intervals, implementation can be achieved with minimal logistic support and by fewer resource personnel • Loss of randomization units during the course of the trial may compromise the integrity of the trial

Thank You sgoudar@jnmc. edu
 Cynchia
Cynchia Rsna clinical trial processor
Rsna clinical trial processor Morpheus bms
Morpheus bms Clinical trial budget example
Clinical trial budget example Novel clinical drug trial design
Novel clinical drug trial design Clinical trials api
Clinical trials api Clinical trial financial management
Clinical trial financial management Drug development timeline
Drug development timeline Nida clinical trials network
Nida clinical trials network Ivd clinical trial design
Ivd clinical trial design Clinical trial exports
Clinical trial exports Clinical trial worksheet
Clinical trial worksheet Master clinical trial agreements
Master clinical trial agreements Clinical trial matching service
Clinical trial matching service Dsp research tracker
Dsp research tracker



























