Alterations in Fluid Electrolyte and AcidBase Balance Lecture

Alterations in Fluid, Electrolyte and Acid-Base Balance Lecture 9 1

Introduction • Fluid is dynamic state. • Body fluid: is body water that has solutes dissolve on it. Some solutes are electrolyte. • Electrolyte such as Na, K, Ca, CL and Mg. 2

Water may serve as: 1. Medium of metabolic reaction with cells. 2. Transporter for nutrients, waste products, and other substance. 3. A lubricant. 4. Shock absorber. 5. Regulate and maintain body temperature. 3

General Concepts • Intake = Output = Fluid Balance • Sensible losses – Urination – Defecation – Wound drainage • Insensible losses – Evaporation from skin – Respiratory loss from lungs 4
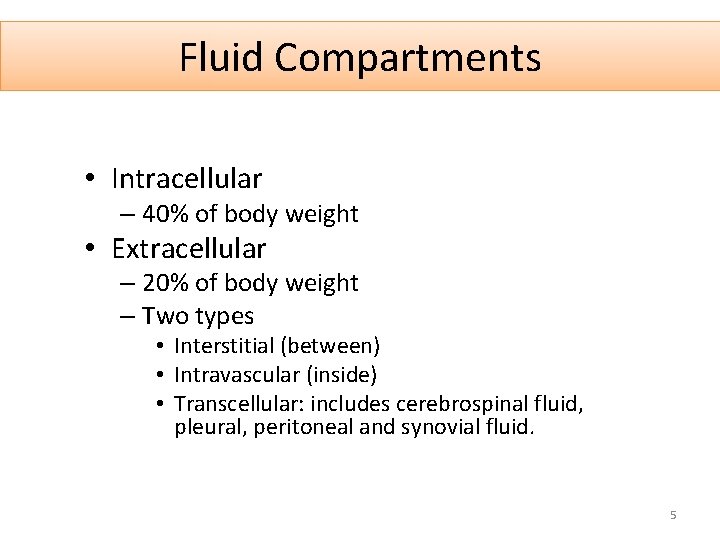
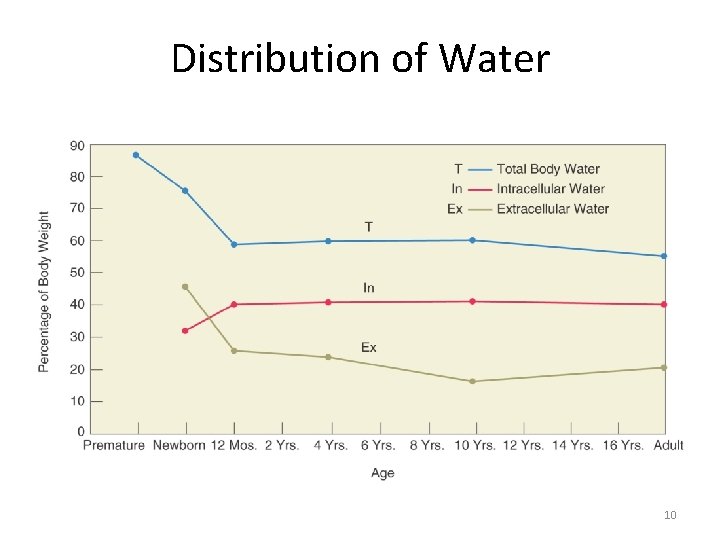
Fluid Compartments • Intracellular – 40% of body weight • Extracellular – 20% of body weight – Two types • Interstitial (between) • Intravascular (inside) • Transcellular: includes cerebrospinal fluid, pleural, peritoneal and synovial fluid. 5
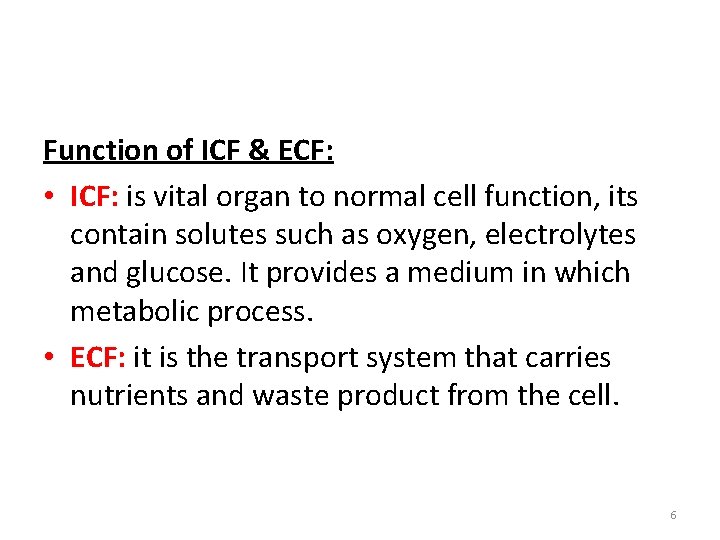
Function of ICF & ECF: • ICF: is vital organ to normal cell function, its contain solutes such as oxygen, electrolytes and glucose. It provides a medium in which metabolic process. • ECF: it is the transport system that carries nutrients and waste product from the cell. 6
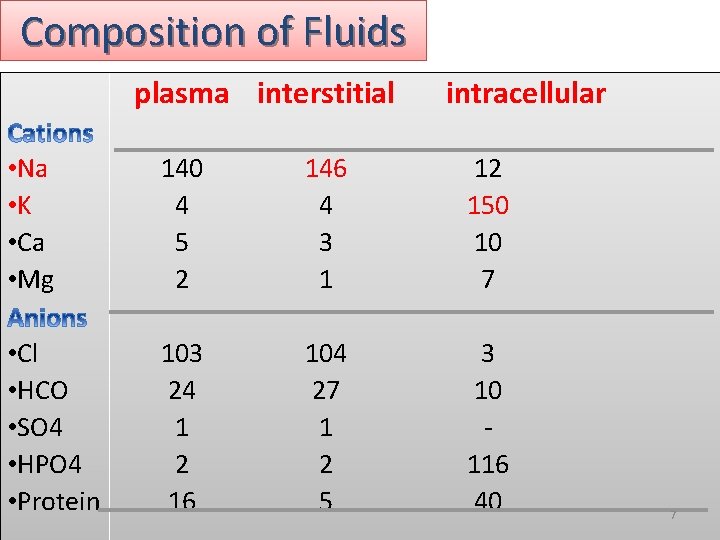
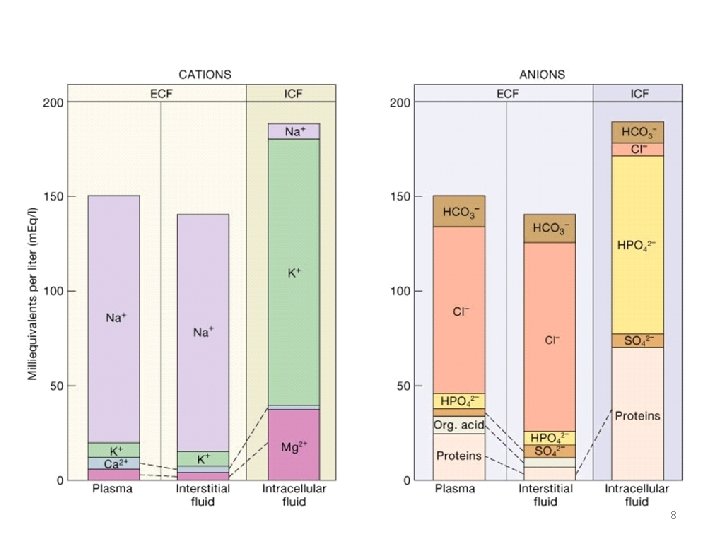
Composition of Fluids plasma interstitial intracellular • Na • K • Ca • Mg 140 4 5 2 146 4 3 1 12 150 10 7 • Cl • HCO • SO 4 • HPO 4 • Protein 103 24 1 2 16 104 27 1 2 5 3 10 116 40 7
8
Pediatric Differences • ECF/ICF ratio varies with age. • Neonates and infants have proportionately larger ECF volume • Infants: high daily fluid requirement with little fluid reserve; this makes the infant vulnerable to dehydration. 9
Distribution of Water 10
Fluid Loss; Infants and <2 yr. excretion is via the urine, feces, lungs and skin have greater daily fluid loss than older child more dependent upon adequate intake greater about of skin surface (BSA), therefore greater insensible loss. • respiratory and metabolic rates are higher therefore, dehydrate more rapidly • • 11
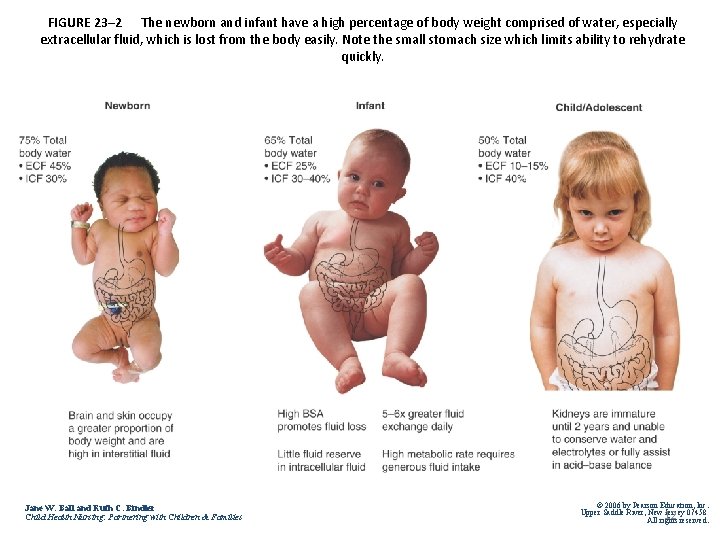
FIGURE 23– 2 The newborn and infant have a high percentage of body weight comprised of water, especially extracellular fluid, which is lost from the body easily. Note the small stomach size which limits ability to rehydrate quickly. Jane W. Ball and Ruth C. Bindler Child Health Nursing: Partnering with Children & Families © 2006 by Pearson Education, Inc. Upper Saddle River, New 12 Jersey 07458 All rights reserved.

Pediatric differences § <2 yr kidneys immature § less able to conserve or excrete water and solutes effectively § Infants have weaker transport system (ion, bicarbonate) greater risk for acid/base imbalances § Difficulty regulating electrolyte such as Na, Ca 13
Fluid and Electrolyte Transport • PASSIVE TRANSPORT SYSTEMS – Diffusion – Filtration – Osmosis • ACTIVE TRANSPORT SYSTEM – Pumping – Requires energy expenditure 14
1. Diffusion • Molecules move across a biological membrane from an area of higher to an area of lower concentration • Membrane types – Permeable – Semi-permeable 15
2. Filtration • Movement of solute and solvent across a membrane caused by hydrostatic (water pushing) pressure • Occurs at the capillary level • If normal pressure gradient changes (as occurs with right-sided heart failure) edema results from “third spacing” 16

3. Osmosis • Movement of solvent from an area of lower solute concentration to one of higher concentration • Occurs through a semipermeable membrane using osmotic (water pulling) pressure 17

– Solutes are substance dissolved in liquid. – Solvent: is the component of solution that can dissolve in the solutes. – Crystalloid: salts that dissolved readily in to true solution. – Colloids: substance such as large protein molecules that do not dissolved in true solution. • Sodium is the major determinant of serum osmolality. 18
4. Active Transport System • Solutes can be moved against a concentration gradient • Also called “pumping” • Dependent on the presence of ATP 19
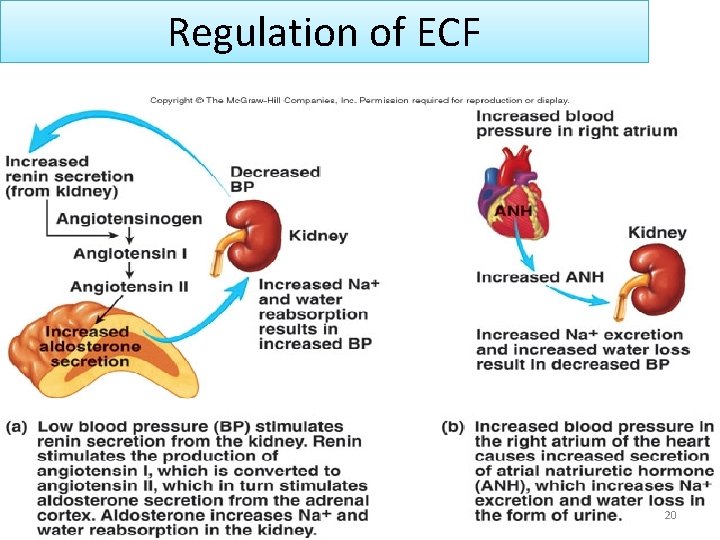
Regulation of ECF 20
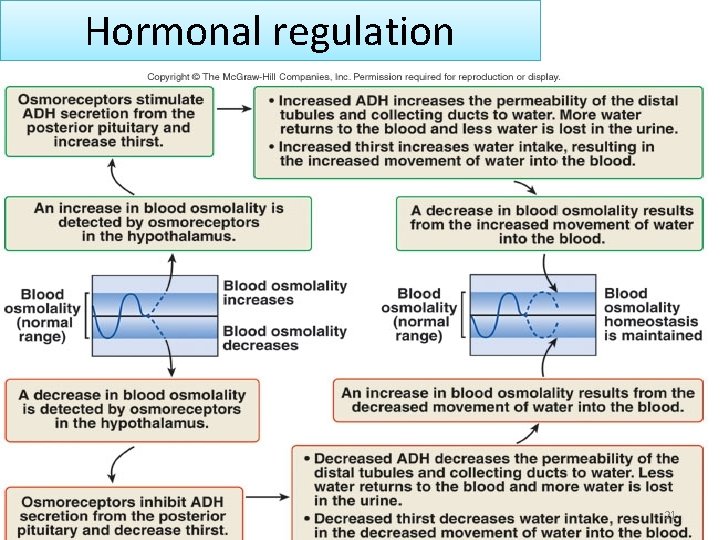
Hormonal regulation 21
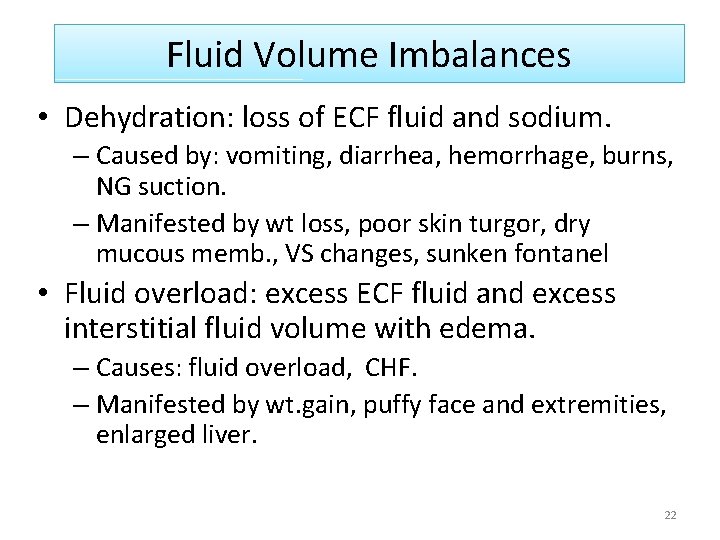
Fluid Volume Imbalances • Dehydration: loss of ECF fluid and sodium. – Caused by: vomiting, diarrhea, hemorrhage, burns, NG suction. – Manifested by wt loss, poor skin turgor, dry mucous memb. , VS changes, sunken fontanel • Fluid overload: excess ECF fluid and excess interstitial fluid volume with edema. – Causes: fluid overload, CHF. – Manifested by wt. gain, puffy face and extremities, enlarged liver. 22

Nursing Considerations • How can the nurse determine if the child is mildly dehydrated vs moderately dehydrated? 23

Mild Dehydration: by history. • hard to detect because the child may be alert, have moist mucous membranes and normal skin turgor. • Wt loss may be up to 5% of body weight. • The infant might be irritable; the older child might be thirsty • vital signs will probably be normal • Capillary refill will most likely be normal • Urine output may be normal or sl less 24
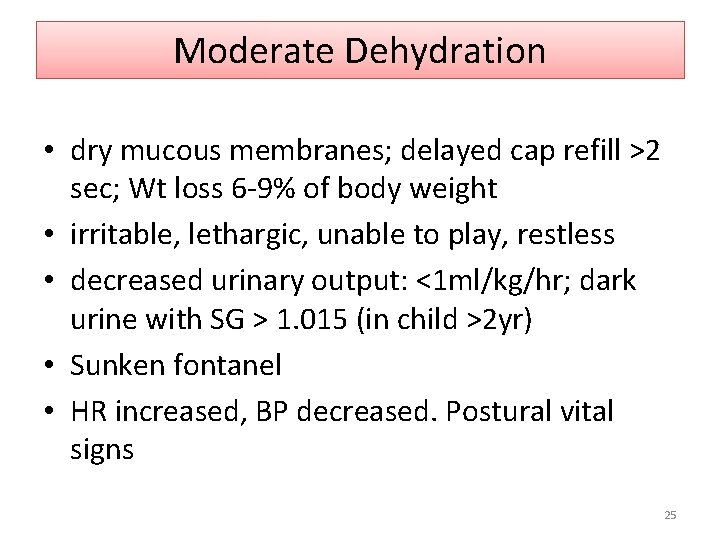
Moderate Dehydration • dry mucous membranes; delayed cap refill >2 sec; Wt loss 6 -9% of body weight • irritable, lethargic, unable to play, restless • decreased urinary output: <1 ml/kg/hr; dark urine with SG > 1. 015 (in child >2 yr) • Sunken fontanel • HR increased, BP decreased. Postural vital signs 25
Severe Dehydration • wt loss > 10% body weight • lethargic/comatose • rapid weak pulse with BP low or undetectable; RR variable and labored. • dry mucous membranes/parched; sunken fontanel • decr or absent urinary output. • Cap refill >4 sec 26
Types of Dehydration and Sodium Loss • Sodium may be: – Low – High – Or normal 27

1. Isotonic Dehydration or Isonatremic Dehydration • Loss of sodium and water are in proportion • Most of fluid lost is from extracellular component • Serum sodium is normal (130 -150 m. Eq/L). – Most practitioners consider below 135 and above 148 a more conservative parameter (138 -148) – Most common form of dehydration in young children from vomiting and diarrhea. 28
2. Hypotonic or Hyponatremic Dehydration • Greater loss of sodium than water • Serum sodium below normal • Compensatory shift of fluids from extracellular to intracellular makes extracellular dehydration worse. • Caused by severe and prolonged vomiting and diarrhea, burns, renal disease. Also by treatment of dehydration with IV fluids without electrolytes. 29

3. Hypertonic or Hypernatremic Dehydration • Greater loss of water than sodium • Serum sodium is elevated • Compensatory shift from intracellular to extracellular which masks the severity of water loss (dehydration) delaying signs and symptoms until condition is quite serious. • Caused by concentrated IV fluids or tube feedings. 30

Etiology of dehydration 1. Vomiting and diarrhea, nasogastric suction and burn. 2. Water loss = under the warmer. 3. Accumulation of fluid in third space. 4. Over use diuretics. 5. Excessive exercise 31
Rotavirus • • Common viral form of diarrhea All ages but 3 mo-2 yrs most common Fecal/oral route Virus remains active; – 10 days on hard, dry surfaces – 4 hrs on human hands – 1 wk on wet areas 32

Rotavirus (cont. ) • Incubation period 1 -3 days • Symptoms: mild/mod fever, stomach ache, frequent watery stools (20/day) • Treatment: prevention! Hand washing and isolation of the infected child. • Fluid rehydration for diarrhea, advanced to bland diet for older children • Breast milk for the infant who BF 33

Clinical Management for Dehydration • Blood may be drawn to assess electrolytes, BUN and Creatinine levels • an IV may be placed the same time • Oral Rehydration Solution is the treatment of choice for mild-moderate dehydration – 1 -3 tsp of ORS every 10 -15 min to start (even if vomits some) – 50 ml/Kg/Hr is the goal for rehydration. 34

Why are drinks high in glucose avoided during rehydration? • Simple sugars increases the osmotic effect in the intestine by pulling water into the colon, thereby increasing diarrhea and subsequent fluid/electrolyte loss • Drinks high in glucose: apple juice, sodas, jello water. 35

Recommended foods during rehydration progression: • starches, cooked fruits & vegetables, soups, yogurt, formula, breast milk. • recent research has shown no difference than return to normal diet with some attention to lactose containing foods, depending upon the child’s response. 36
IV Therapy • Used for severe dehydration or in the child who will not/cannot tolerate ORS • 24 hr maintenance plus replacement given within first 6 -8 hr (in ER) to rapidly expand the intravascular space. Usually a normal saline bolus. • slower IV rate for the remainder of the first 24 hrs • nurse records IV volume infused hourly 37

Which of the following IV solutions replaces Sodium? 1. 2. 3. 4. D 5 W Lactated Ringers Normal Saline D 5 ½ NS 38
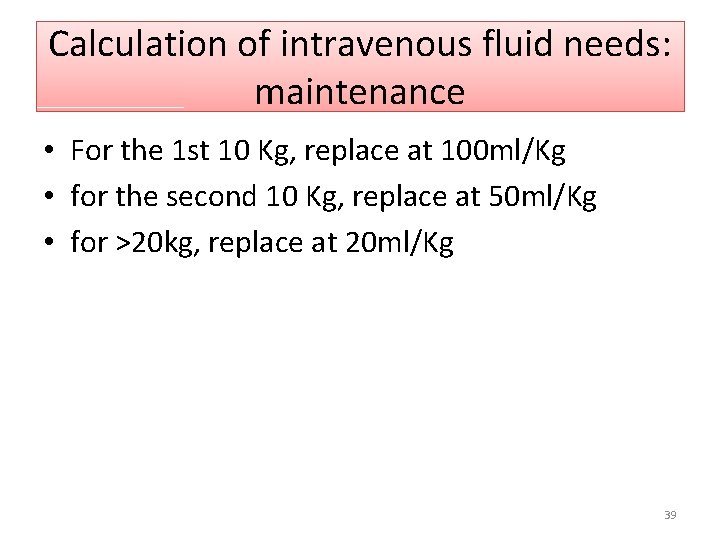
Calculation of intravenous fluid needs: maintenance • For the 1 st 10 Kg, replace at 100 ml/Kg • for the second 10 Kg, replace at 50 ml/Kg • for >20 kg, replace at 20 ml/Kg 39
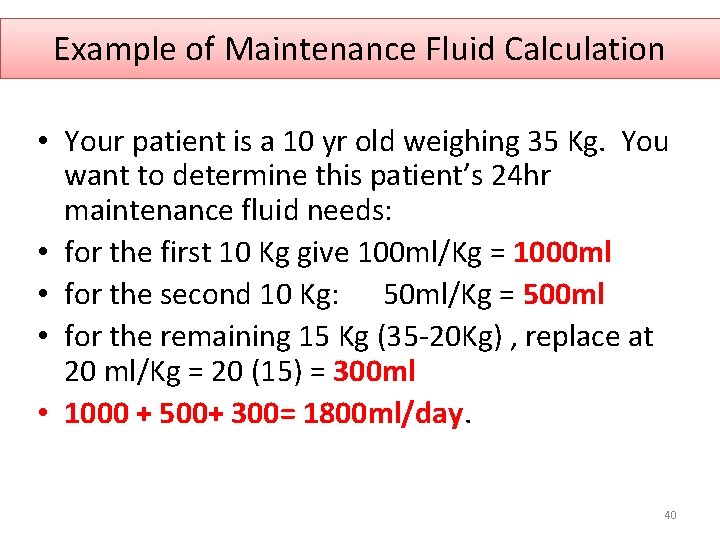
Example of Maintenance Fluid Calculation • Your patient is a 10 yr old weighing 35 Kg. You want to determine this patient’s 24 hr maintenance fluid needs: • for the first 10 Kg give 100 ml/Kg = 1000 ml • for the second 10 Kg: 50 ml/Kg = 500 ml • for the remaining 15 Kg (35 -20 Kg) , replace at 20 ml/Kg = 20 (15) = 300 ml • 1000 + 500+ 300= 1800 ml/day. 40

How much fluid should this patient get per hour? • 1800 ml / 24 hrs = 75 ml/hr. • Therefore, if the patient were NPO and not taking in fluids from any other source, the IV should be running at 75 ml/hr. • If there is a deficit that also needs to be replaced, the IV rate may be slightly higher for a defined period of time. • If the patient is receiving fluids from other sources, these need to be accounted as well 41
Fluid Overload: Edema • Increase capillary blood flow: inflammation, infection • venous congestion: ECF excess, Rt sided heart failure, muscle paralysis. • Increase albumin excess: Nephrotic Syndrome • Decrease albumin synthesis: Kwashiorkor, liver cirrhosis • Increase capillary permeability: inflam/ burns • blocked lymphatic drainage: tumors/surgury. 42
Assessment/Management of Edema ascites; periorbital edema pitting edema for degree of swelling daily wt and strickt I and O elevation/change position Q 2 hr/ protect skin against breakdown • distraction to deal with discomfort and limitations of edema. • • 43
Electrolyte Imbalances • Electrolytes usually gained and lost in relatively equal amounts to maintain balance • Imbalance caused by: – Abnormal route of loss (vomiting/diarrhea) can disturb electrolyte balance – Disproportionate IV supplementation – Disease states: renal dis. 44
Hypernatremia • Excess serum sodium in relation to water • >146 mmol/l • Causes: – Too concentrated infant formula – Not enough water intake • Clinical manif: thirst, lethary, confusion – Seizures occur when rapid or is severe. – Lab test: serum sodium – Treatment: hypotonic IV solution 45

Hyponatremia • • Excess water in relation to serum sodium < 135 mmol/l Most common sodium imbalance in children Causes: – Infants vulnerable to water intoxication: dilute form, poorly developed thirst mech so cont to drink and can’t excrete excess water or IV fluid. 46
Hyponatremia (cont) • Clinical manif: decreased level of consciousness, swelling of brain cells. – Anorexia, headache, muscle weakness, lethargy, confusion or coma. – Seizures occur when rapid or severe. – Lab tests: serum sodium • Treatment: hypertonic solution. 47
Hyperkalemia • Excess serum potassium > 5. 3 meq/l • Causes: – excess K intake from IV overload, blood transfusion, rapid cell death (hemolytic crisis, large tumor destruction from chemo , massive trauma, metabolic acidosis from prolonged diarrhea and in DM when insulin levels are low • Insulin drives K back into the cells – decreased K loss from Renal insufficiency 48
Hyperkalemia (cont) • Clinical manif: all are related to muscle dysfunction: hyperactivitiy of GI smooth muscle: intestinal cramping and diarrhea. – Weak skeletal muscles – Lethargy – Cardiac arrhythmias (tachycardia, prolonged QRS, peaked T waves: also AV block and venticular Tachy). – Lab test: serum potassium – Treatment: correct underlying condition – dialysis (peritoneal or hemo), Kayexalate (po or enema), K wasting diuretics, IV calcium, bicarbonate, insulin and glucose. – Low potassium diet. 49
Hypokalemia • Decreased serum potassium < 3. 5 meq/l • Causes: diarrhea and vomiting, diuretics, osmotic diuresis (glucose in urine as in DM), NPO without K replacement in IV, NG Suction, – Also in nephrotic syndrome, cirrhosis, Cushing Syndrome, CHF 50
Hypokalemia (cont) • Clinical manif: muscle dysfunction • Slowed GI smooth muscle resulting in abdominal distention, constipation and paralytic ileus • Skeletal muscles are weak; may effect respiratory muscles • Cardiac arrhythmias: hypokalemia potentiates Digitoxin Toxicity. • Lab test: serum potassium • Treatment: oral and/or IV potassium, diet rich in K. 51
Hypercalcemia • Excess calcium 5 meq/l • Needs vit D for efficient absorption; most of Ca is stored in the bones. • Causes: bone tumors that cause bone destruction, chemo theraoy release Ca from the bones; immobilization causes loss from the bones (usually excreted) but if kidneys can’t clear it, hypercalcemia results, increased intake. 52
Hypercalcemia (cont) • Clinical manif: Ca imbalances alter neuromuscular irritability with non-specific symptoms – Constipation, anorexia, N/V, fatigue, skeletal muscle weakness, confusion, lethargy. – Renal calculi, cardiac arrhythmias – Hyper. Ca increases Na and K excretion leading to polyuria and polydipsia. – Rx: serum Ca, Ionized Ca, fluids, Lasix, steroids, dialysis. 53
Hypocalcemia • Decreased serum calcium < 4 meq/l • Causes: decreased intake of Ca and/or Vit D – Limited exposure to sunlight, premature infants and dark skinned people at increased risk to inadeq. Vit D and therefore decreased Ca absorption. – Parathyroid dysfunction, multiple transfusion (Citrate binds Calcium), steatorrhea (as in pancreatitis) binds Calcium in the stool. 54
Hypocalcemia (cont) • Clinical Manif: acute situation related to increased muscular excitability: tetany. • In children: cramping, tingling around the mouth or fingers. • In infants: tremors, muscle twitches, brief tonic-clonic seizures, CHF. • Laryngospasm, seizures and cardiac arrhythmias in severe situations. 55
Hypocalcemia (cont 2) • In children and adolescents, chronic hypocalcemia more common, manif. By spontaneous fractures. Lab tests: serum Ca; bone density study Rx: oral and/or IV Ca, Ca rich diet 56

Acid Base Balance • Normal arterial blood p. H: 7. 35 -7. 43 (in general) • Acidosis < 7. 35 : too much acid • Alkalosis > 7. 45 : too little acid • p. CO 2 reflects carbonic acid status: 40 +- 5 • HCO 3 - reflects metabolic acid status: 24 +- 4 57
Respiratory Acidosis • • caused by decrease respiratory effort build up of CO 2 in the blood p. H decr or normal; p. CO 2 incr. Symptoms manifested: confusion, lethargy, increase ICP, coma, tachycardia, arrhythmias 58
Management of Respiratory Acidosis • • Incr ventilatory rate give O 2 intubate adm Na. HCO 3 59
Respiratory Alkalosis • • caused by hyperventilation CO 2 is being blown off p. H incr : p. Co 2 decr Symptoms: dizziness, confusion, neuromuscular irritability, paresthesias in extremities and circumoral, muscle cramping. 60

Management of Resp. Alkalosis • First determine if oxygenation is adequate, if not, you don’t want to slow the RR. • Determine the cause and correct it: – Causes of hypervent: hypoxemia, anxiety, pain, fever, ASA toxicity, meningitis/encephalitis, Gram - sepsis, mechanical overventilation. 61
Metabolic Acidosis • • • caused by a loss of bicarbonate (HCO 3) therefore, is an incr of acids in the blood p. H decr or moving towards normal p. Co 2 decr ; HCO 3 decr Symptoms: Kussmaul respirations = incr rate and depth as compensation (hyperventilation/acetone breath), confusion, hypotension, tissue hypoxia, cardiac arrhythmias, pulmonary edema. 62

Management of Metabolic Acidosis • Identify and treat underlying cause • In severe case may give IV Na. HCO 3 to incr p. H, or insulin/glucose. • Causes: ingestion of ASA, antifreeze, oliguria, lactic acidosis (tissue hypoxia). • Loss of HCO 3: urine disease, diarrhea, Renal failure. 63

Metabolic Alkalosis • caused by loss of H+ or HCO 3 retention • HCO 3 incr with probable incr in p. H, incr p. CO 2. • Symptoms: weak, dizzy, muscle cramps, twitching, tremors, slow shallow resp. , disorientation, seizures. 64
Management of Metabolic Alkalosis • correct underlying cause; facilitate renal excretion of HCO 3. • admin NS, K+ if hypokalemic, replace loss of fluids, monitor I and O and electrolytes • Causes: prolonged vomiting, antacids, loss of NG fluids, hypokalemia from prolonged diuretic use, multiple blood transfusion with citrate. 65
ABG Basic (Uncompensated) Analysis • Resp Acidosis: low p. H and high Pa. CO 2 • Resp Alkalosis: incr p. H and low Pa. CO 2 • Metab Acidosis: low p. H and nl Pa. Co 2; decr HCO 3 • Metab Alkalosis: high p. H; nl Pa. CO 2 ; high HCO 3 66
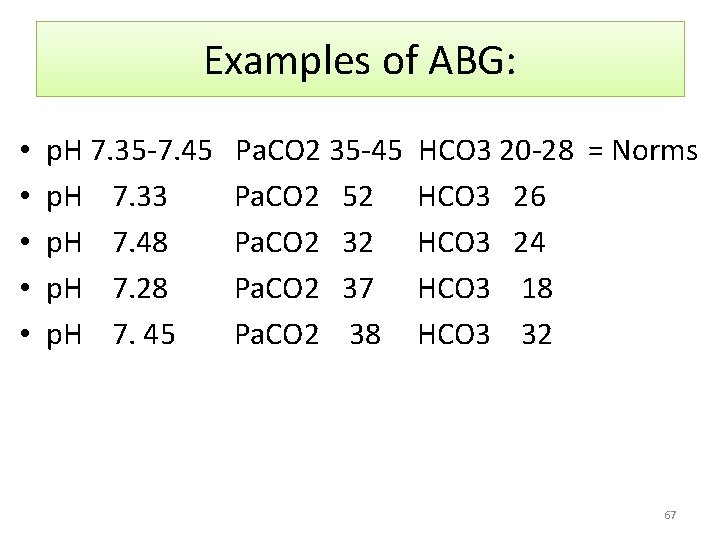
Examples of ABG: • • • p. H 7. 35 -7. 45 p. H 7. 33 p. H 7. 48 p. H 7. 28 p. H 7. 45 Pa. CO 2 35 -45 Pa. CO 2 52 Pa. CO 2 37 Pa. CO 2 38 HCO 3 20 -28 = Norms HCO 3 26 HCO 3 24 HCO 3 18 HCO 3 32 67
- Slides: 67