Alpha Decay parent nucleus Atomic number 2 mass
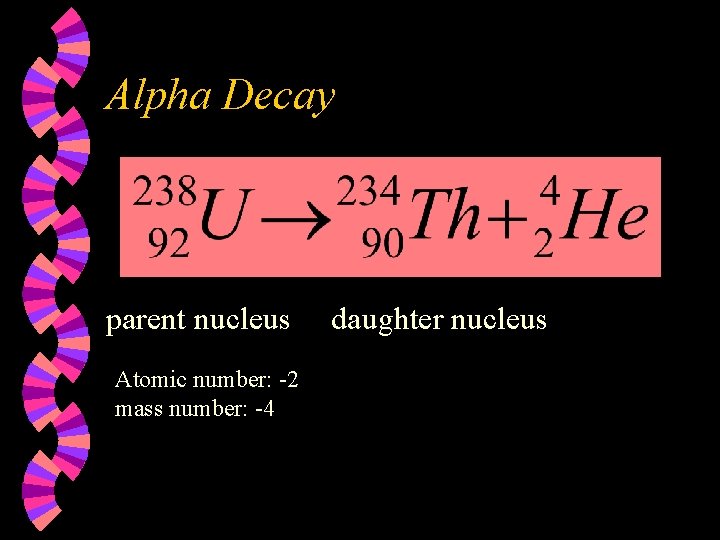
Alpha Decay parent nucleus Atomic number: -2 mass number: -4 daughter nucleus
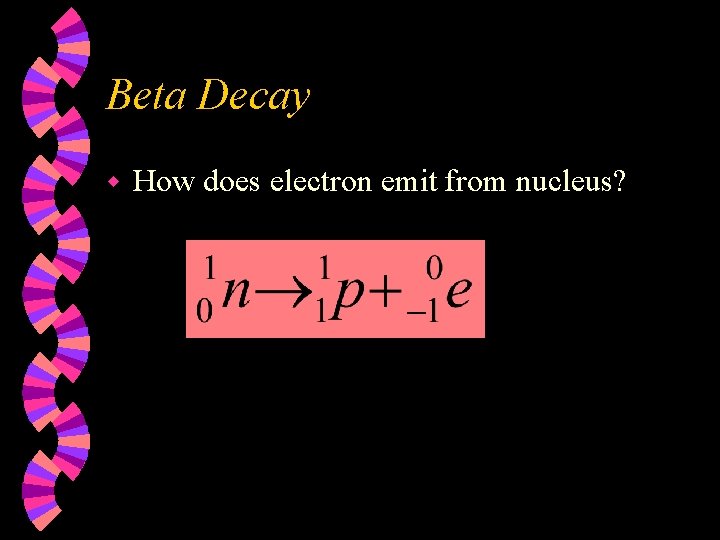
Beta Decay w How does electron emit from nucleus?
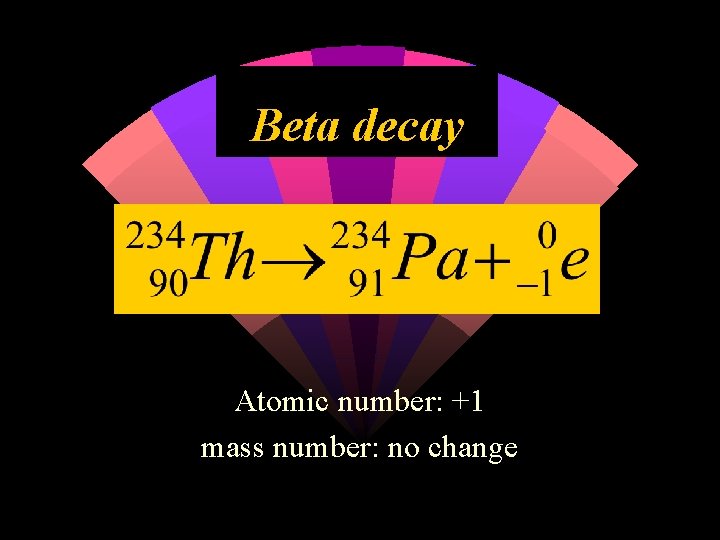
Beta decay Atomic number: +1 mass number: no change

Gamma Waves are emitted from the nucleus w atomic number and mass number no change w
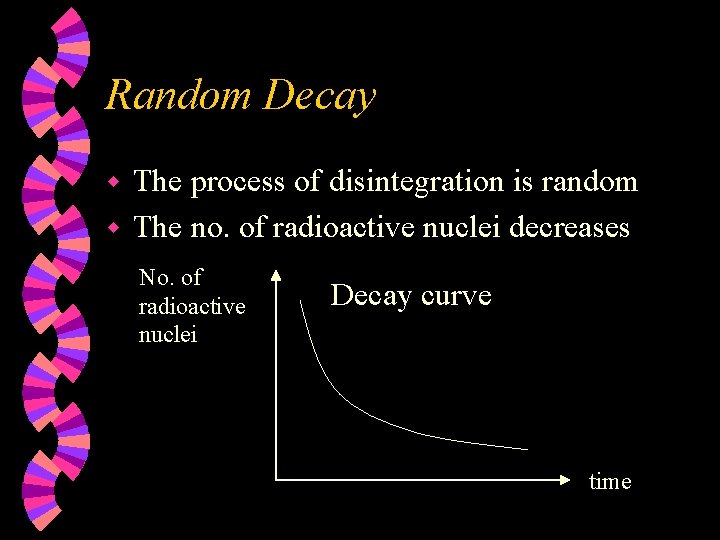
Random Decay The process of disintegration is random w The no. of radioactive nuclei decreases w No. of radioactive nuclei Decay curve time
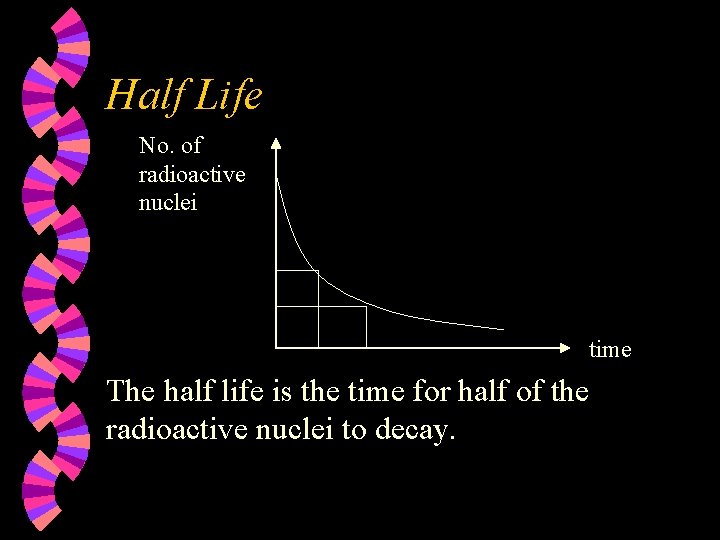
Half Life No. of radioactive nuclei time The half life is the time for half of the radioactive nuclei to decay.

Half Life w w w Radon-220 Sodium-24 Iodine-131 Carbon-14 Uranium-235 52 s 15 hours 8 days 5600 years 70000 years

Activity When the no. of radioactive nuclei decreases, the rate of radiation emission also decreases. w Activity = no. of disintegration per second w unit : Bq (becquerel) w

Use of Radioisotope w w w Radiotherapy Tracer Sterilisation Thickness gauge Smoke detector Carbon dating

Radiotherapy Kill cancer cells w Circular treatment w Use gamma: high penetrating power w

Tracer Inject radioactive source into body w Let the body absorb the radioactive source w Detect the radiation outside body w • e. g. Iodine-131 to detect thyroid gland Sodium-24 to detect blood clot Use gamma or beta: penetrate through body w Short half life: several hours to several days w

Sterilisation Use radiation to kill bacteria in food w Sterilize syringes w Use gamma ray: high penetrating power w

Thickness Gauge Check thickness of materials w Use beta ray w If too thin, more beta particle penetrate through and the count rate increase w If too thick, less beta particle penetrate through and the count rate decrease w

Smoke Detector Radioactive source produces ions in both chamber w two equal current produced w If there is fire, more ions enter the open chamber. Two current not equal. w Alarm on. w

Carbon-14 Dating Every living things contain C-12 and C-14 with specific ratio. w When living things died, C-14 decays and become less and less. w Ratio of C-12 and C-14 can be used in archaeological dating. w

Use of Radioisotope w w w w Radiotherapy Tracer Sterilization Thickness gauge Smoke detector Carbon dating Nuclear energy

Nuclear Fission When neutron bombard uranium nucleus, it splits into smaller parts. w Energy is released in the process. w This is called fission. w 1 kg uranium releases 10000000 J w
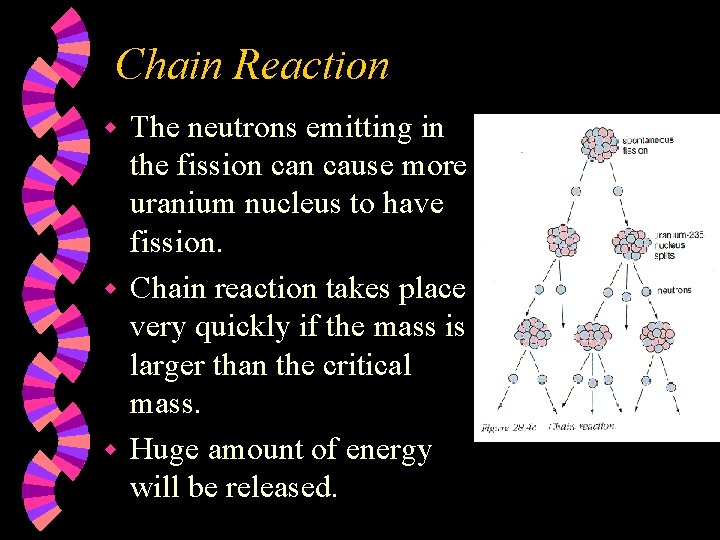
Chain Reaction The neutrons emitting in the fission cause more uranium nucleus to have fission. w Chain reaction takes place very quickly if the mass is larger than the critical mass. w Huge amount of energy will be released. w

Atomic Bomb vs Nuclear Reactor w Atomic Bomb: uncontrolled fission w Nuclear reactor: controlled fission • fission heat up water • hot water boils secondary water • steam turns turbine of generator
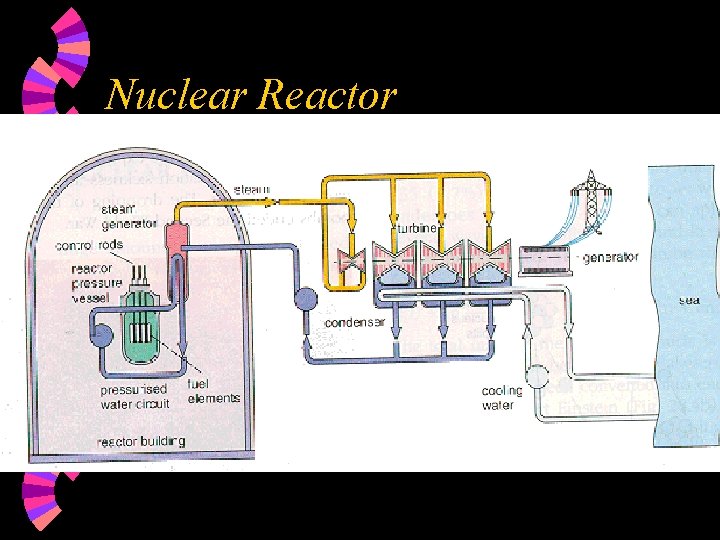
Nuclear Reactor

Nuclear Reactor Fuel element: uranium rod w moderator: w • keep neutron at suitable speed of fission (slow speed) w control rod • absorb neutrons • control rate of reaction

Safety of Nuclear Reactor Thick reactor building w Thick steel reactor w Control rod w Use pressurized water to avoid boiling w w Monitor nearby surrounding’s background radiation.

Fusion w When two light nuclei join together to form heavy nucleus, energy is released. This is called fusion. w 1 kg of hydrogen gives 60000000 J. w

Fusion’s Adv. And Disadv. w Advantage: • many fuel • less pollution • more energy released w Disadvantage: • requires very high temperature (like the Sun) • difficult to find container w Uncontrolled fusion: hydrogen bomb • Start by atomic bomb

Nuclear Energy Debate w Pros • Solve world’s energy problem • Cheaper than coal or oil • Less pollution than coal or oil w Cons • • Unacceptable hazard if exploded Many nuclear waste Lead to nuclear weapon, nuclear war Other better energy resources available
- Slides: 25