ALLY2 Study DCV SOF for HCV in HIV
- Slides: 8

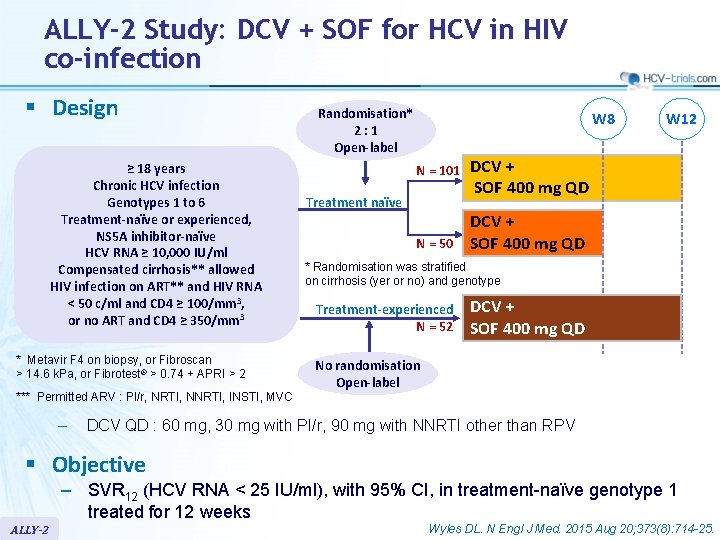
ALLY-2 Study: DCV + SOF for HCV in HIV co-infection § Design ≥ 18 years Chronic HCV infection Genotypes 1 to 6 Treatment-naïve or experienced, NS 5 A inhibitor-naïve HCV RNA ≥ 10, 000 IU/ml Compensated cirrhosis** allowed HIV infection on ART** and HIV RNA < 50 c/ml and CD 4 ≥ 100/mm 3, or no ART and CD 4 ≥ 350/mm 3 * Metavir F 4 on biopsy, or Fibroscan > 14. 6 k. Pa, or Fibrotest® > 0. 74 + APRI > 2 Randomisation* 2: 1 Open-label W 8 N = 101 Treatment naïve N = 50 W 12 DCV + SOF 400 mg QD * Randomisation was stratified on cirrhosis (yer or no) and genotype Treatment-experienced N = 52 DCV + SOF 400 mg QD No randomisation Open-label *** Permitted ARV : PI/r, NRTI, NNRTI, INSTI, MVC – DCV QD : 60 mg, 30 mg with PI/r, 90 mg with NNRTI other than RPV § Objective – SVR 12 (HCV RNA < 25 IU/ml), with 95% CI, in treatment-naïve genotype 1 treated for 12 weeks ALLY-2 Wyles DL. N Engl J Med. 2015 Aug 20; 373(8): 714 -25.

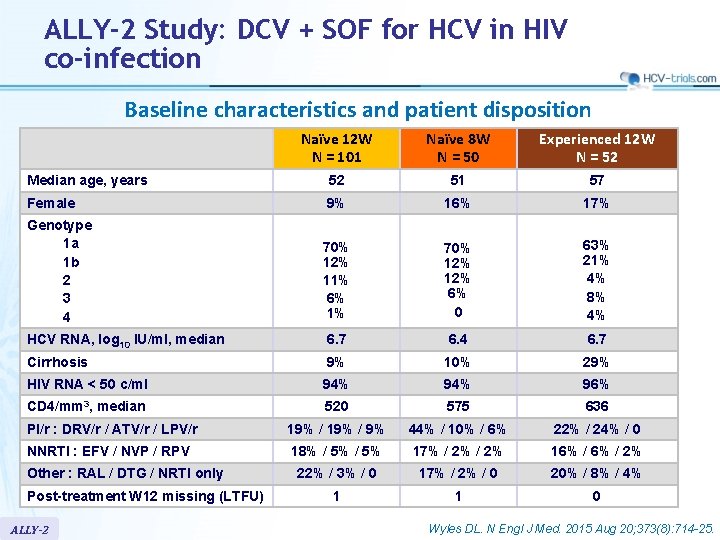
ALLY-2 Study: DCV + SOF for HCV in HIV co-infection Baseline characteristics and patient disposition Naïve 12 W N = 101 Naïve 8 W N = 50 Experienced 12 W N = 52 Median age, years 52 51 57 Female 9% 16% 17% Genotype 1 a 1 b 2 3 4 70% 12% 11% 6% 1% 70% 12% 6% 0 63% 21% 4% 8% 4% HCV RNA, log 10 IU/ml, median 6. 7 6. 4 6. 7 Cirrhosis 9% 10% 29% HIV RNA < 50 c/ml 94% 96% CD 4/mm 3, median 520 575 636 PI/r : DRV/r / ATV/r / LPV/r 19% / 9% 44% / 10% / 6% 22% / 24% / 0 NNRTI : EFV / NVP / RPV 18% / 5% 17% / 2% 16% / 2% 22% / 3% / 0 17% / 2% / 0 20% / 8% / 4% 1 1 0 Other : RAL / DTG / NRTI only Post-treatment W 12 missing (LTFU) ALLY-2 Wyles DL. N Engl J Med. 2015 Aug 20; 373(8): 714 -25.

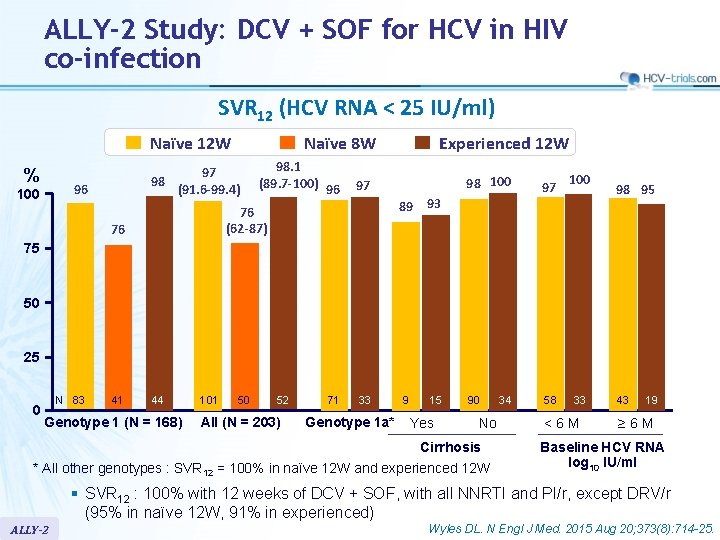
ALLY-2 Study: DCV + SOF for HCV in HIV co-infection SVR 12 (HCV RNA < 25 IU/ml) Naïve 12 W % 97 98 (91. 6 -99. 4) 96 100 Naïve 8 W 98. 1 (89. 7 -100) 96 97 98 100 97 90 58 89 93 76 (62 -87) 76 Experienced 12 W 100 98 95 75 50 25 0 N 83 41 44 Genotype 1 (N = 168) 101 50 52 All (N = 203) 71 33 Genotype 1 a* 9 15 Yes No Cirrhosis * All other genotypes : SVR 12 = 100% in naïve 12 W and experienced 12 W 34 33 <6 M 43 19 ≥ 6 M Baseline HCV RNA log 10 IU/ml § SVR 12 : 100% with 12 weeks of DCV + SOF, with all NNRTI and PI/r, except DRV/r (95% in naïve 12 W, 91% in experienced) ALLY-2 Wyles DL. N Engl J Med. 2015 Aug 20; 373(8): 714 -25.

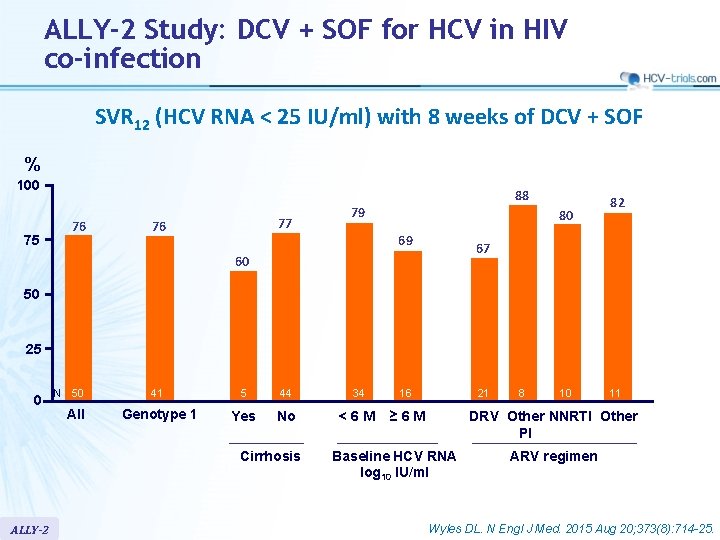
ALLY-2 Study: DCV + SOF for HCV in HIV co-infection SVR 12 (HCV RNA < 25 IU/ml) with 8 weeks of DCV + SOF % 100 75 88 76 77 76 79 80 69 82 67 60 50 25 0 N 50 All 41 5 44 34 16 Genotype 1 Yes No <6 M ≥ 6 M Cirrhosis ALLY-2 21 8 10 11 DRV Other NNRTI Other PI Baseline HCV RNA log 10 IU/ml ARV regimen Wyles DL. N Engl J Med. 2015 Aug 20; 373(8): 714 -25.

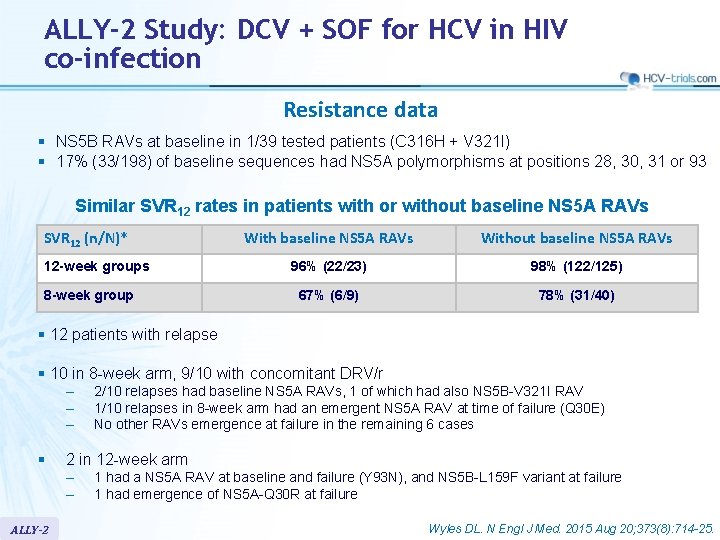
ALLY-2 Study: DCV + SOF for HCV in HIV co-infection Resistance data § NS 5 B RAVs at baseline in 1/39 tested patients (C 316 H + V 321 I) § 17% (33/198) of baseline sequences had NS 5 A polymorphisms at positions 28, 30, 31 or 93 Similar SVR 12 rates in patients with or without baseline NS 5 A RAVs SVR 12 (n/N)* 12 -week groups 8 -week group With baseline NS 5 A RAVs Without baseline NS 5 A RAVs 96% (22/23) 98% (122/125) 67% (6/9) 78% (31/40) § 12 patients with relapse § 10 in 8 -week arm, 9/10 with concomitant DRV/r – – – § 2 in 12 -week arm – – ALLY-2 2/10 relapses had baseline NS 5 A RAVs, 1 of which had also NS 5 B-V 321 I RAV 1/10 relapses in 8 -week arm had an emergent NS 5 A RAV at time of failure (Q 30 E) No other RAVs emergence at failure in the remaining 6 cases 1 had a NS 5 A RAV at baseline and failure (Y 93 N), and NS 5 B-L 159 F variant at failure 1 had emergence of NS 5 A-Q 30 R at failure Wyles DL. N Engl J Med. 2015 Aug 20; 373(8): 714 -25.

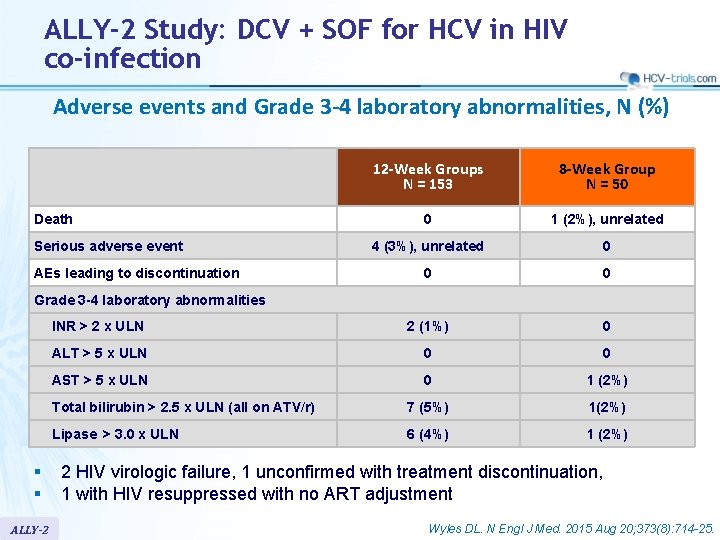
ALLY-2 Study: DCV + SOF for HCV in HIV co-infection Adverse events and Grade 3 -4 laboratory abnormalities, N (%) 12 -Week Groups N = 153 8 -Week Group N = 50 0 1 (2%), unrelated 4 (3%), unrelated 0 0 0 INR > 2 x ULN 2 (1%) 0 ALT > 5 x ULN 0 0 AST > 5 x ULN 0 1 (2%) Total bilirubin > 2. 5 x ULN (all on ATV/r) 7 (5%) 1(2%) Lipase > 3. 0 x ULN 6 (4%) 1 (2%) Death Serious adverse event AEs leading to discontinuation Grade 3 -4 laboratory abnormalities § § ALLY-2 2 HIV virologic failure, 1 unconfirmed with treatment discontinuation, 1 with HIV resuppressed with no ART adjustment Wyles DL. N Engl J Med. 2015 Aug 20; 373(8): 714 -25.

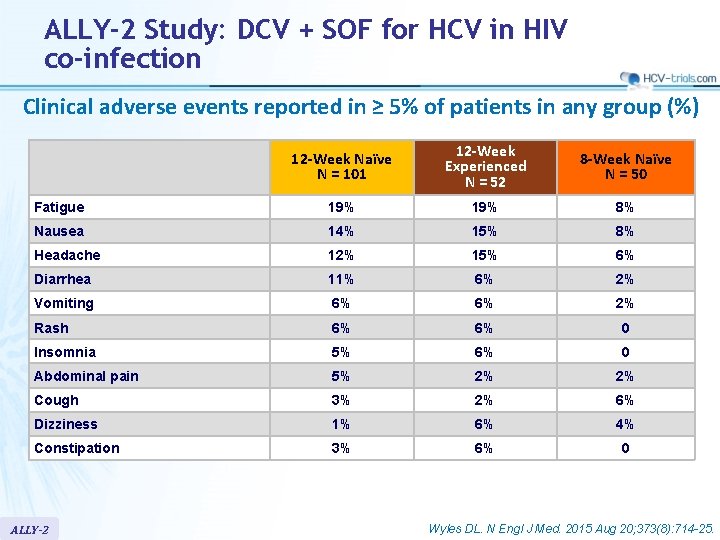
ALLY-2 Study: DCV + SOF for HCV in HIV co-infection Clinical adverse events reported in ≥ 5% of patients in any group (%) 12 -Week Naïve N = 101 12 -Week Experienced N = 52 8 -Week Naïve N = 50 Fatigue 19% 8% Nausea 14% 15% 8% Headache 12% 15% 6% Diarrhea 11% 6% 2% Vomiting 6% 6% 2% Rash 6% 6% 0 Insomnia 5% 6% 0 Abdominal pain 5% 2% 2% Cough 3% 2% 6% Dizziness 1% 6% 4% Constipation 3% 6% 0 ALLY-2 Wyles DL. N Engl J Med. 2015 Aug 20; 373(8): 714 -25.

ALLY-2 Study: DCV + SOF for HCV in HIV co-infection § Summary – After 12 weeks of DCV + SOF in HIV/HCV coinfected patients, the overall SVR 12 was 97% • 97% in GT 1 ; 100% in GT 2, 3, and 4 • 97% in treatment-naive and 98% in treatment-experienced patients • No significant effect of race, baseline HCV RNA levels, cirrhosis or ARV regimen – In patients treated for 8 weeks with DCV + SOF, SVR 12 was 76% – Increased relapse in coinfected patients with shorter therapy, higher baseline HCV RNA (≥ 2 M IU/ml), and DRV/r-based c. ART with DCV 30 mg QD – No compromise of HIV suppression and no modification of ontreatment ARV regimens due to DCV + SOF – DCV + SOF was safe and well tolerated, offers a predictable drug interaction profile with flexibility to dose adjust, and is compatible with a wide range of antiretrovirals ALLY-2 Wyles DL. N Engl J Med. 2015 Aug 20; 373(8): 714 -25.