Allosteric Enzyme ATCase Active relaxed form Catalytic subunits
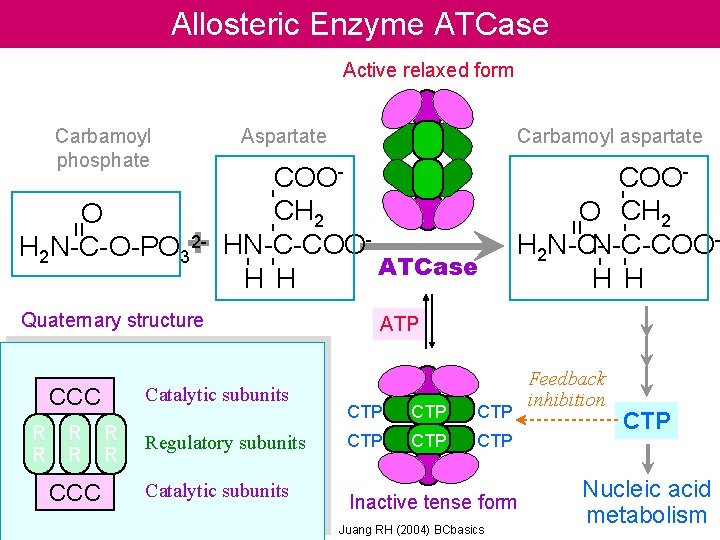
Allosteric Enzyme ATCase Active relaxed form Catalytic subunits R R CCC R R Regulatory subunits Catalytic subunits COOO CH 2 N-C-COOH 2 N-CH H - - - COOCH 2 HN-C-COOATCase H H Quaternary structure CCC Carbamoyl aspartate = = O 2 H 2 N-C-O-PO 3+ Aspartate - - - Carbamoyl phosphate ATP CTP CTP CTP Inactive tense form Juang RH (2004) BCbasics Feedback inhibition CTP Nucleic acid metabolism
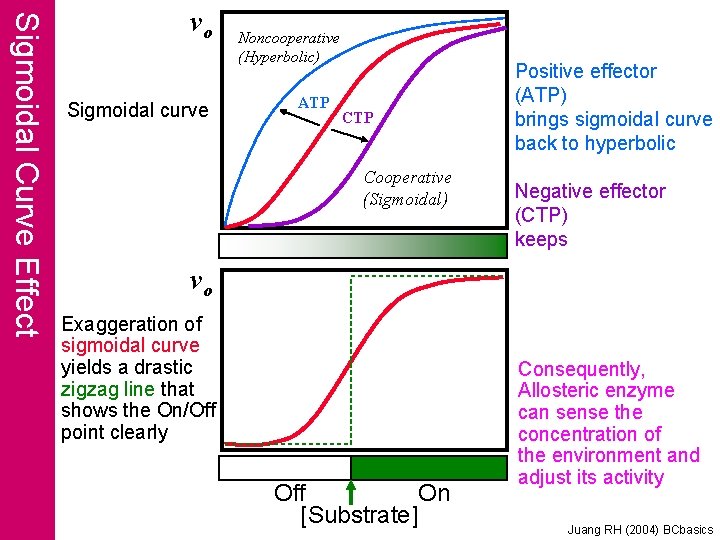
Sigmoidal Curve Effect vo Sigmoidal curve Noncooperative (Hyperbolic) ATP Cooperative (Sigmoidal) Positive effector (ATP) brings sigmoidal curve back to hyperbolic Negative effector (CTP) keeps vo Exaggeration of sigmoidal curve yields a drastic zigzag line that shows the On/Off point clearly Off On [Substrate] Consequently, Allosteric enzyme can sense the concentration of the environment and adjust its activity Juang RH (2004) BCbasics
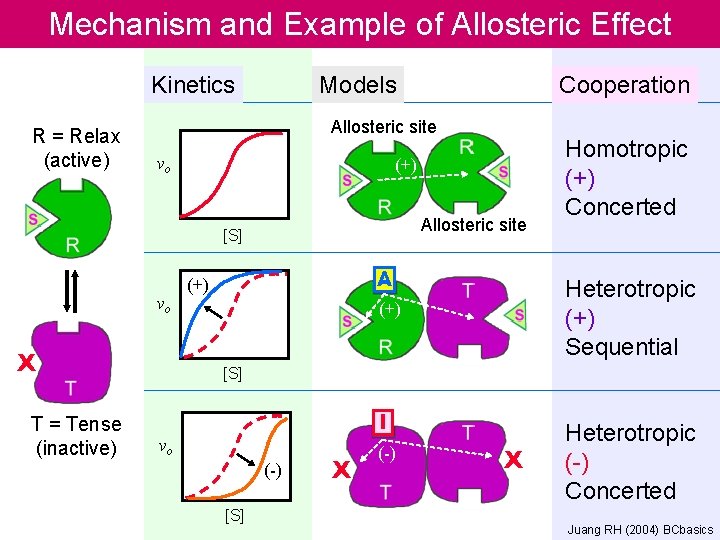
Mechanism and Example of Allosteric Effect Kinetics R = Relax (active) Models Allosteric site vo (+) Allosteric site [S] vo X T = Tense (inactive) Cooperation A (+) Homotropic (+) Concerted Heterotropic (+) Sequential (+) [S] I vo (-) [S] X (-) X Heterotropic (-) Concerted Juang RH (2004) BCbasics
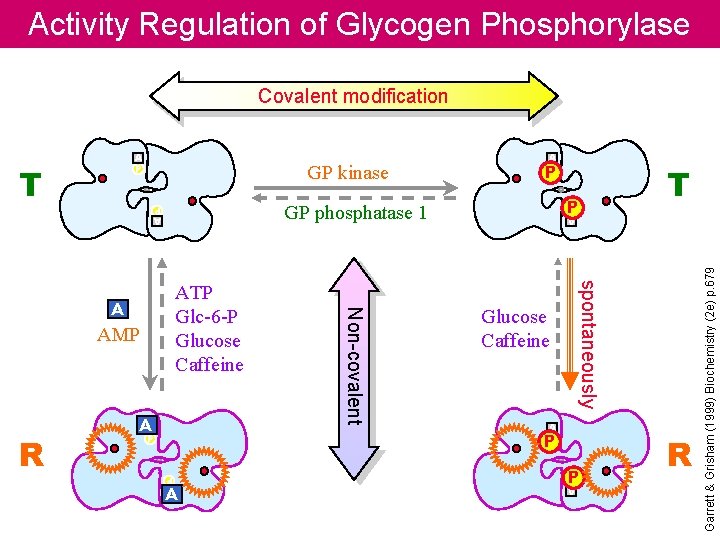
Activity Regulation of Glycogen Phosphorylase Covalent modification P GP phosphatase 1 A P AA Glucose Caffeine A P P A P A P P T spontaneously AMP Non-covalent ATP Glc-6 -P Glucose Caffeine A R P A P T GP kinase A P R Garrett & Grisham (1999) Biochemistry (2 e) p. 679 A P
- Slides: 4