ALL organisms contain and use the same basic
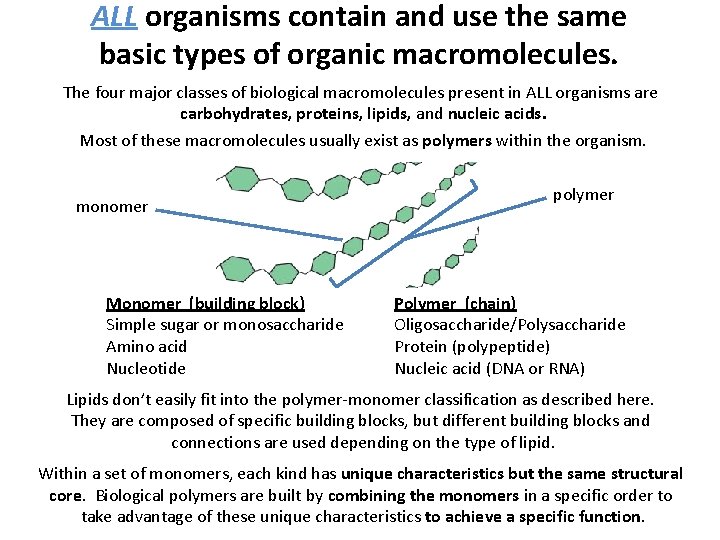
ALL organisms contain and use the same basic types of organic macromolecules. The four major classes of biological macromolecules present in ALL organisms are carbohydrates, proteins, lipids, and nucleic acids. Most of these macromolecules usually exist as polymers within the organism. monomer Monomer (building block) Simple sugar or monosaccharide Amino acid Nucleotide polymer Polymer (chain) Oligosaccharide/Polysaccharide Protein (polypeptide) Nucleic acid (DNA or RNA) Lipids don’t easily fit into the polymer-monomer classification as described here. They are composed of specific building blocks, but different building blocks and connections are used depending on the type of lipid. Within a set of monomers, each kind has unique characteristics but the same structural core. Biological polymers are built by combining the monomers in a specific order to take advantage of these unique characteristics to achieve a specific function.
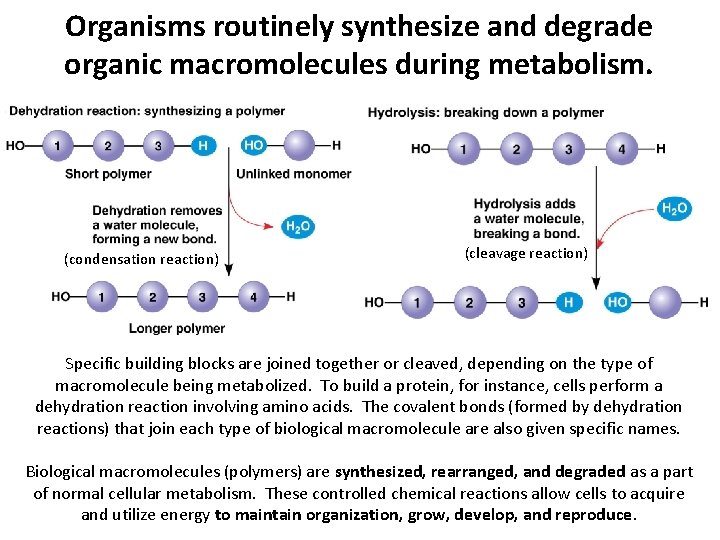
Organisms routinely synthesize and degrade organic macromolecules during metabolism. (condensation reaction) (cleavage reaction) Specific building blocks are joined together or cleaved, depending on the type of macromolecule being metabolized. To build a protein, for instance, cells perform a dehydration reaction involving amino acids. The covalent bonds (formed by dehydration reactions) that join each type of biological macromolecule are also given specific names. Biological macromolecules (polymers) are synthesized, rearranged, and degraded as a part of normal cellular metabolism. These controlled chemical reactions allow cells to acquire and utilize energy to maintain organization, grow, develop, and reproduce.
Carbohydrates are primarily used for energy, structure, and signaling in organisms. Carbohydrates contain carbon, hydrogen, and oxygen atoms in a ratio of 1: 2: 1. Glucose: C 6 H 12 O 6 Producers make carbohydrates by utilizing energy from sunlight (photosynthesis). Consumers acquire carbohydrates by consuming them when they eat producers (usually plants) and/or other consumers that feed on producers. Three main biological functions of carbohydrates energy source, structural material, signaling/recognition* (*when attached to other biomolecules like proteins and lipids)
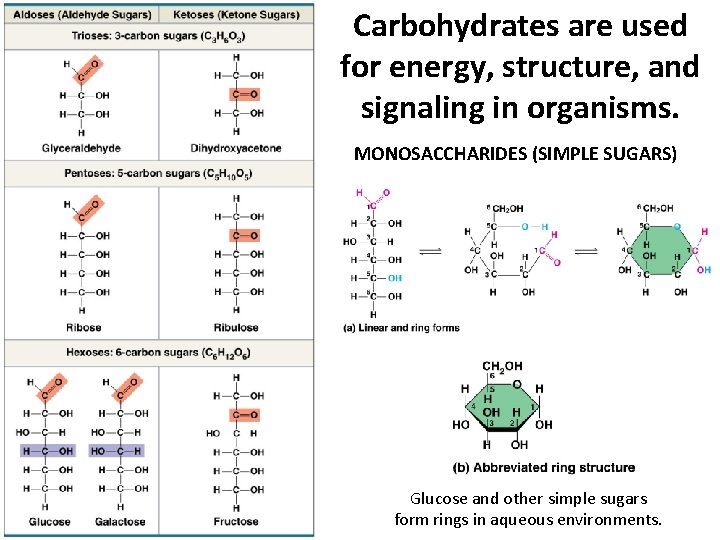
Carbohydrates are used for energy, structure, and signaling in organisms. MONOSACCHARIDES (SIMPLE SUGARS) Glucose and other simple sugars form rings in aqueous environments.
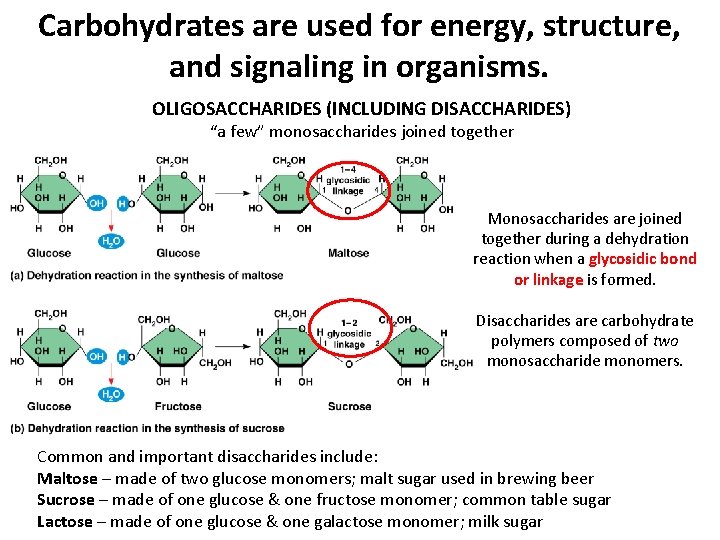
Carbohydrates are used for energy, structure, and signaling in organisms. OLIGOSACCHARIDES (INCLUDING DISACCHARIDES) “a few” monosaccharides joined together Monosaccharides are joined together during a dehydration reaction when a glycosidic bond or linkage is formed. Disaccharides are carbohydrate polymers composed of two monosaccharide monomers. Common and important disaccharides include: Maltose – made of two glucose monomers; malt sugar used in brewing beer Sucrose – made of one glucose & one fructose monomer; common table sugar Lactose – made of one glucose & one galactose monomer; milk sugar
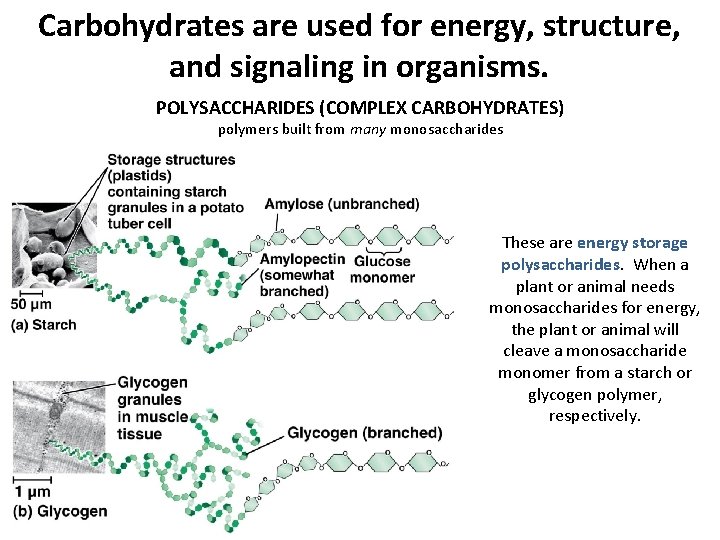
Carbohydrates are used for energy, structure, and signaling in organisms. POLYSACCHARIDES (COMPLEX CARBOHYDRATES) polymers built from many monosaccharides These are energy storage polysaccharides. When a plant or animal needs monosaccharides for energy, the plant or animal will cleave a monosaccharide monomer from a starch or glycogen polymer, respectively.
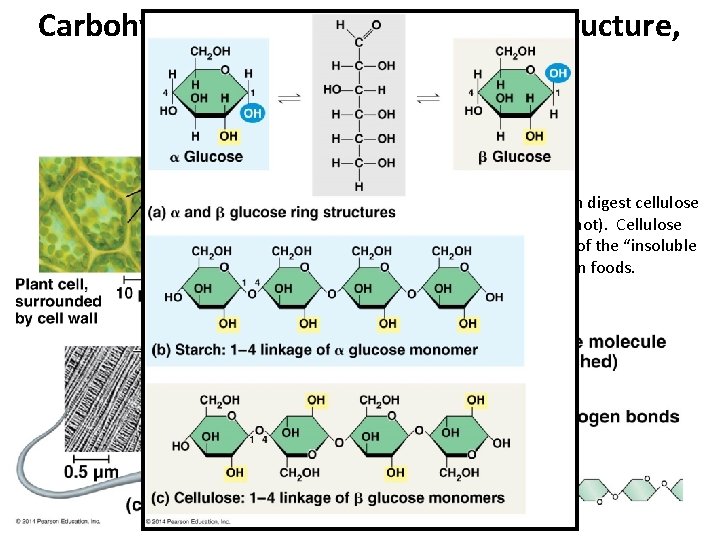
Carbohydrates are used for energy, structure, and signaling in organisms. POLYSACCHARIDES (COMPLEX CARBOHYDRATES) polymers built from many monosaccharides Cellulose is a common plant structural material found in plant and algal cell walls. Few animals can digest cellulose (humans cannot). Cellulose makes up a lot of the “insoluble fiber” in foods.
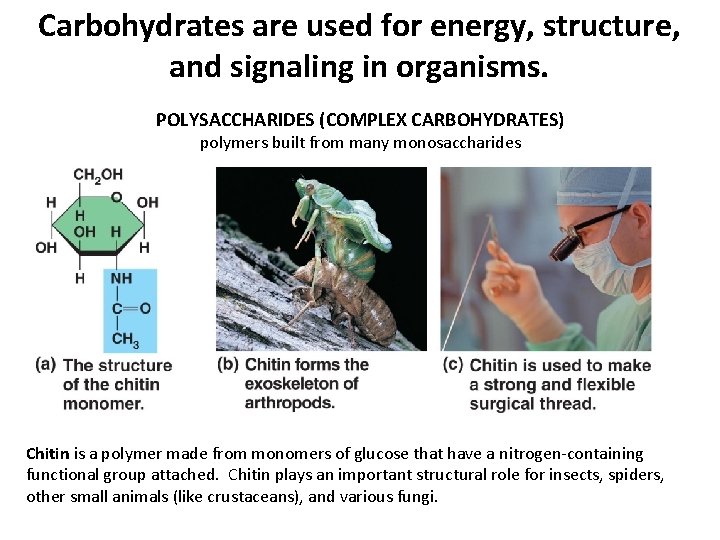
Carbohydrates are used for energy, structure, and signaling in organisms. POLYSACCHARIDES (COMPLEX CARBOHYDRATES) polymers built from many monosaccharides Chitin is a polymer made from monomers of glucose that have a nitrogen-containing functional group attached. Chitin plays an important structural role for insects, spiders, other small animals (like crustaceans), and various fungi.
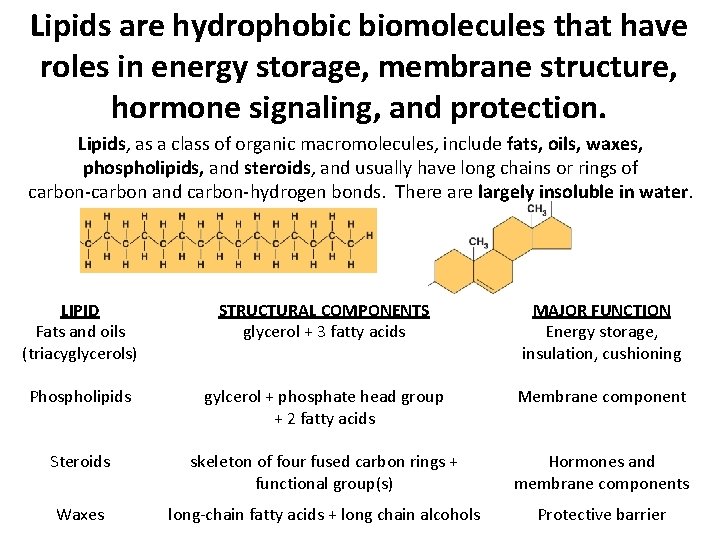
Lipids are hydrophobic biomolecules that have roles in energy storage, membrane structure, hormone signaling, and protection. Lipids, as a class of organic macromolecules, include fats, oils, waxes, phospholipids, and steroids, and usually have long chains or rings of carbon-carbon and carbon-hydrogen bonds. There are largely insoluble in water. LIPID Fats and oils (triacyglycerols) STRUCTURAL COMPONENTS glycerol + 3 fatty acids MAJOR FUNCTION Energy storage, insulation, cushioning Phospholipids gylcerol + phosphate head group + 2 fatty acids Membrane component Steroids skeleton of four fused carbon rings + functional group(s) Hormones and membrane components Waxes long-chain fatty acids + long chain alcohols Protective barrier
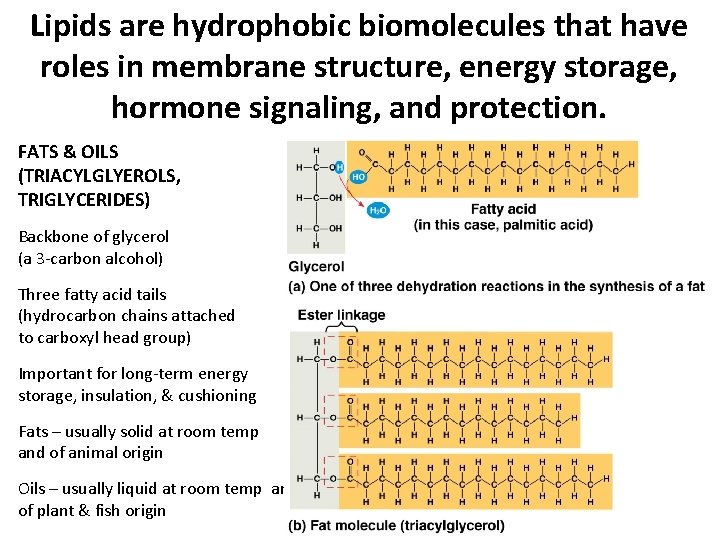
Lipids are hydrophobic biomolecules that have roles in membrane structure, energy storage, hormone signaling, and protection. FATS & OILS (TRIACYLGLYEROLS, TRIGLYCERIDES) Backbone of glycerol (a 3 -carbon alcohol) Three fatty acid tails (hydrocarbon chains attached to carboxyl head group) Important for long-term energy storage, insulation, & cushioning Fats – usually solid at room temp and of animal origin Oils – usually liquid at room temp and of plant & fish origin
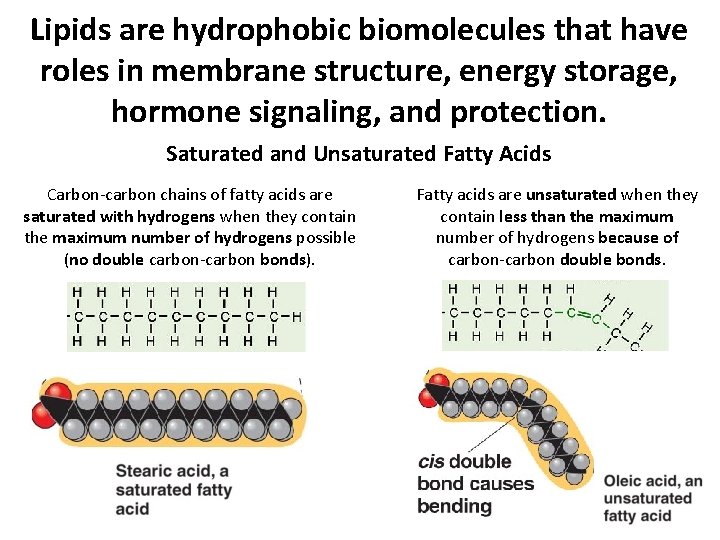
Lipids are hydrophobic biomolecules that have roles in membrane structure, energy storage, hormone signaling, and protection. Saturated and Unsaturated Fatty Acids Carbon-carbon chains of fatty acids are saturated with hydrogens when they contain the maximum number of hydrogens possible (no double carbon-carbon bonds). Fatty acids are unsaturated when they contain less than the maximum number of hydrogens because of carbon-carbon double bonds.
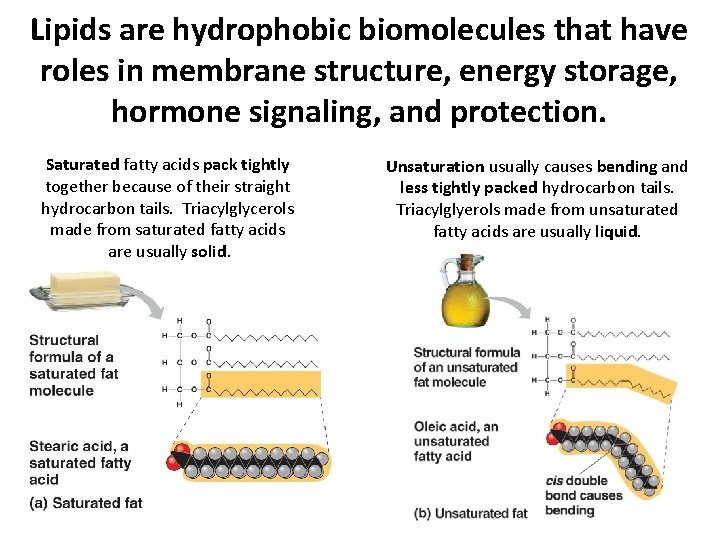
Lipids are hydrophobic biomolecules that have roles in membrane structure, energy storage, hormone signaling, and protection. Saturated fatty acids pack tightly together because of their straight hydrocarbon tails. Triacylglycerols made from saturated fatty acids are usually solid. Unsaturation usually causes bending and less tightly packed hydrocarbon tails. Triacylglyerols made from unsaturated fatty acids are usually liquid.
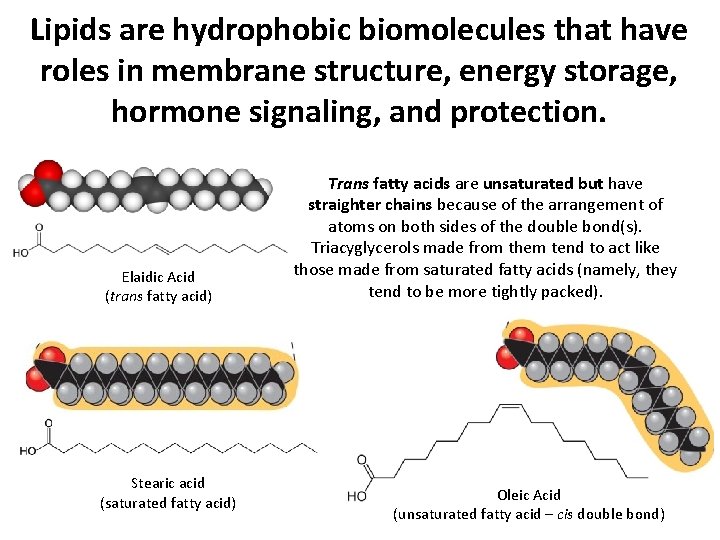
Lipids are hydrophobic biomolecules that have roles in membrane structure, energy storage, hormone signaling, and protection. Elaidic Acid (trans fatty acid) Stearic acid (saturated fatty acid) Trans fatty acids are unsaturated but have straighter chains because of the arrangement of atoms on both sides of the double bond(s). Triacyglycerols made from them tend to act like those made from saturated fatty acids (namely, they tend to be more tightly packed). Oleic Acid (unsaturated fatty acid – cis double bond)
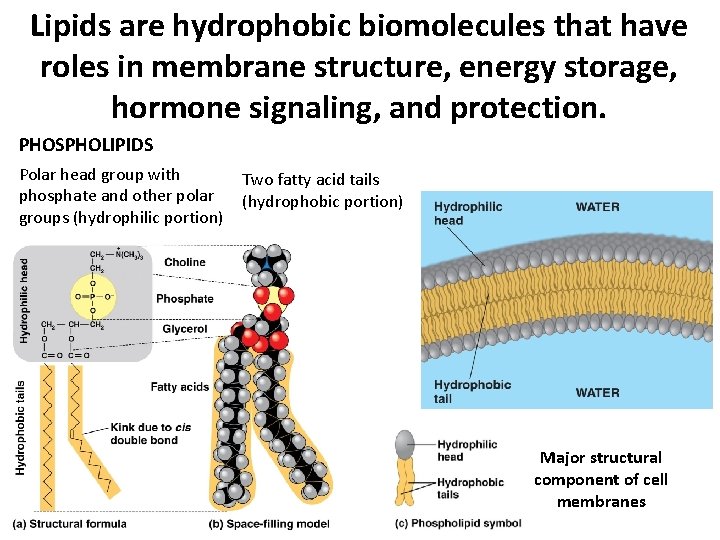
Lipids are hydrophobic biomolecules that have roles in membrane structure, energy storage, hormone signaling, and protection. PHOSPHOLIPIDS Polar head group with phosphate and other polar groups (hydrophilic portion) Two fatty acid tails (hydrophobic portion) Major structural component of cell membranes
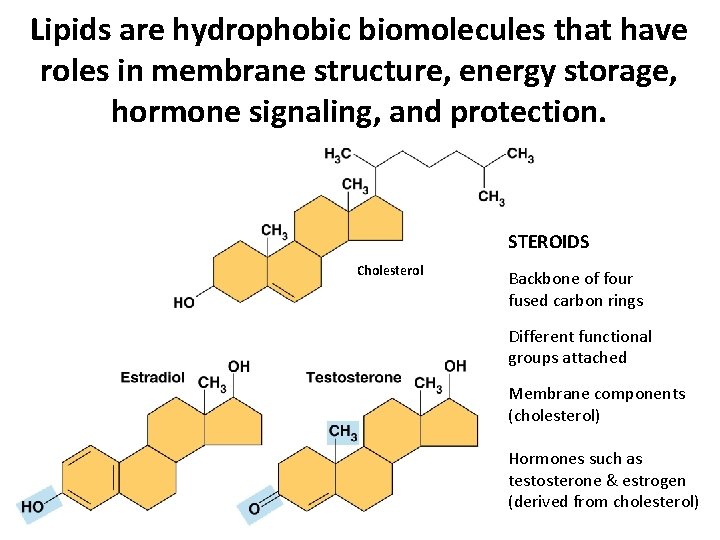
Lipids are hydrophobic biomolecules that have roles in membrane structure, energy storage, hormone signaling, and protection. STEROIDS Cholesterol Backbone of four fused carbon rings Different functional groups attached Membrane components (cholesterol) Hormones such as testosterone & estrogen (derived from cholesterol)
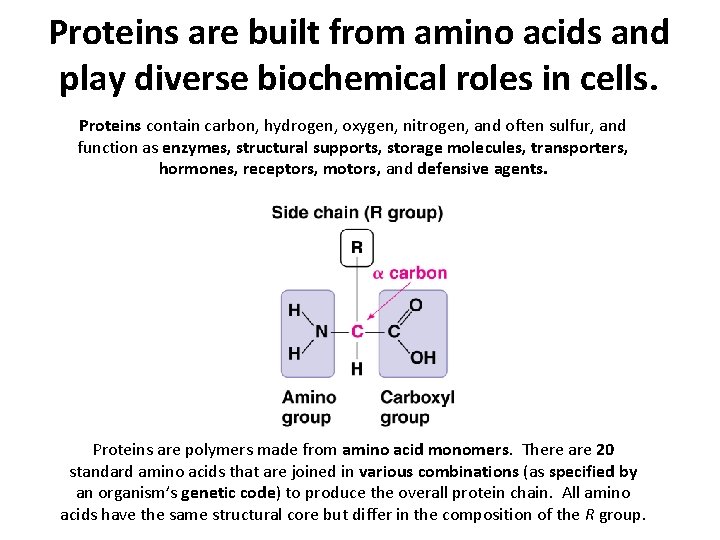
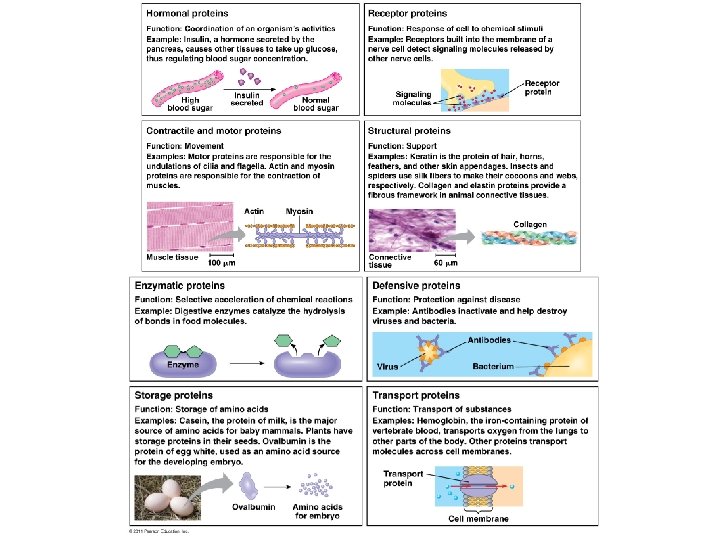
Proteins are built from amino acids and play diverse biochemical roles in cells. Proteins contain carbon, hydrogen, oxygen, nitrogen, and often sulfur, and function as enzymes, structural supports, storage molecules, transporters, hormones, receptors, motors, and defensive agents. Proteins are polymers made from amino acid monomers. There are 20 standard amino acids that are joined in various combinations (as specified by an organism’s genetic code) to produce the overall protein chain. All amino acids have the same structural core but differ in the composition of the R group.
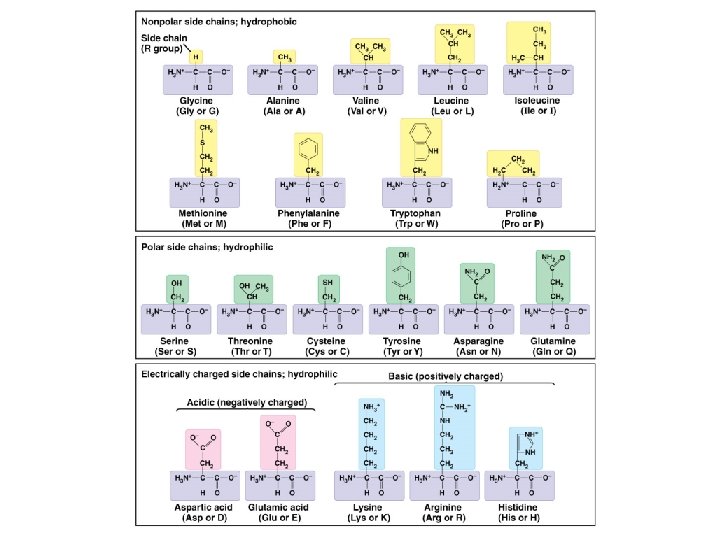
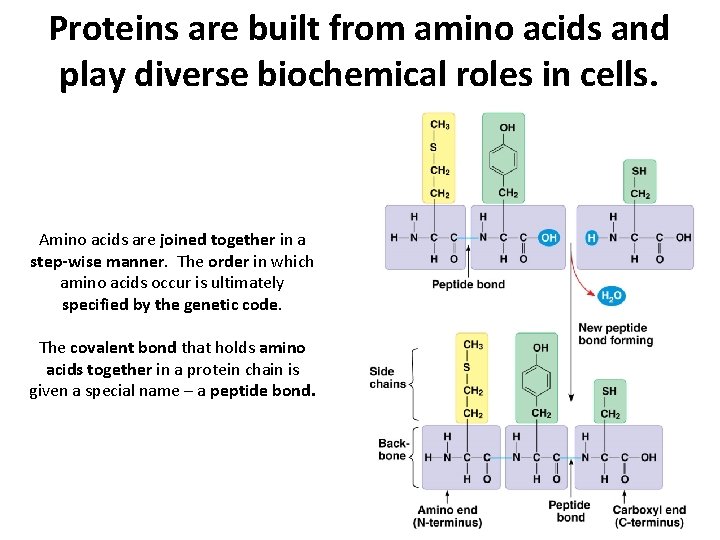
Proteins are built from amino acids and play diverse biochemical roles in cells. Amino acids are joined together in a step-wise manner. The order in which amino acids occur is ultimately specified by the genetic code. The covalent bond that holds amino acids together in a protein chain is given a special name – a peptide bond.
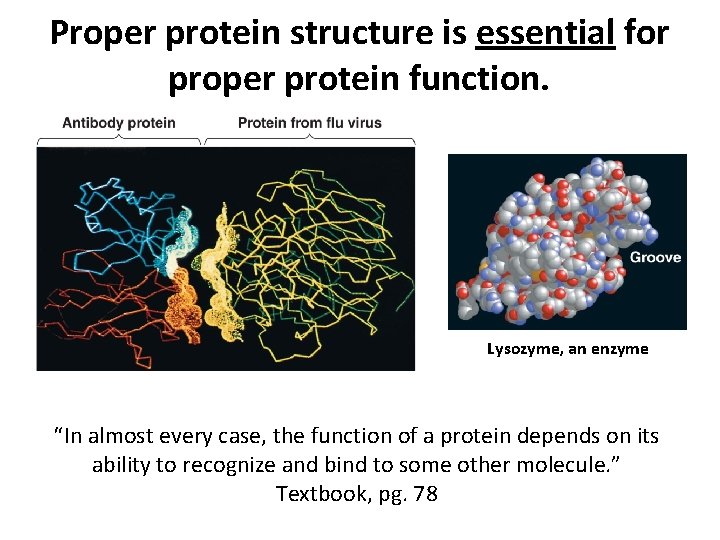
Proper protein structure is essential for proper protein function. Lysozyme, an enzyme “In almost every case, the function of a protein depends on its ability to recognize and bind to some other molecule. ” Textbook, pg. 78
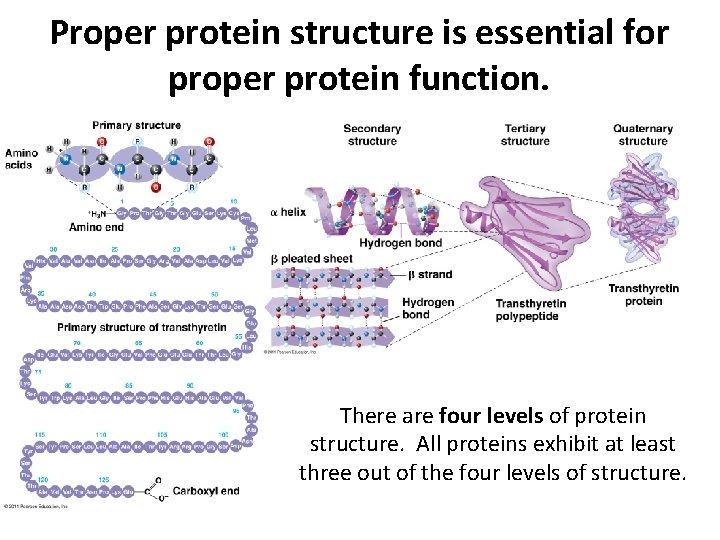
Proper protein structure is essential for proper protein function. There are four levels of protein structure. All proteins exhibit at least three out of the four levels of structure.
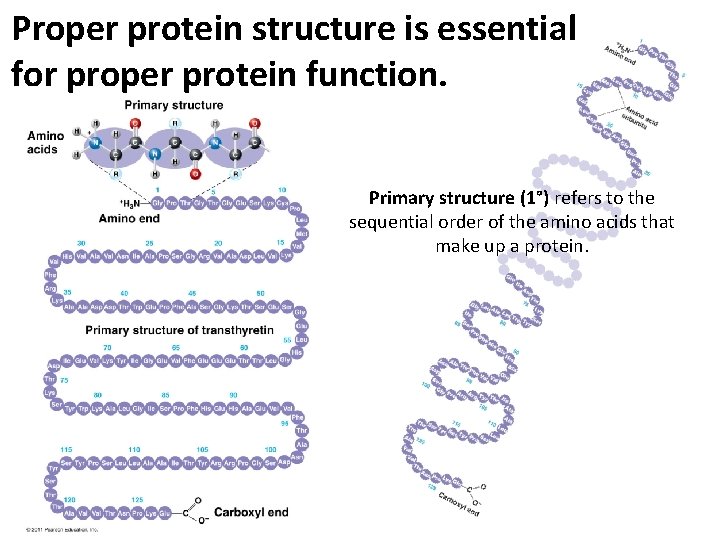
Proper protein structure is essential for proper protein function. Primary structure (1°) refers to the sequential order of the amino acids that make up a protein.
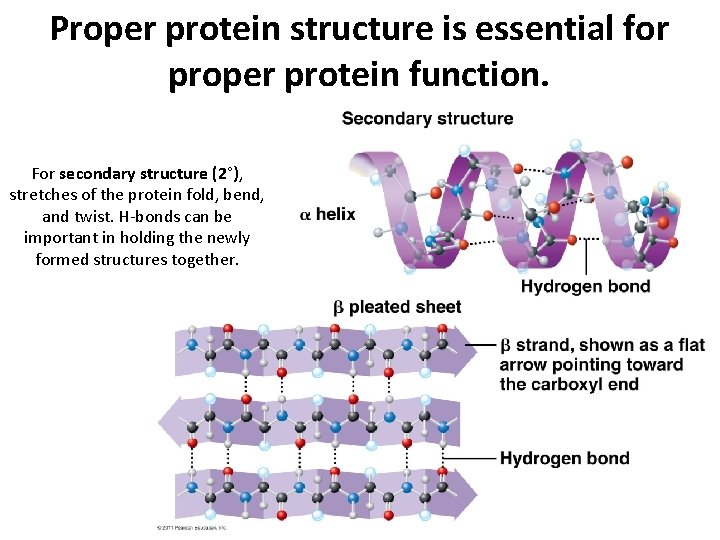
Proper protein structure is essential for proper protein function. For secondary structure (2°), stretches of the protein fold, bend, and twist. H-bonds can be important in holding the newly formed structures together.
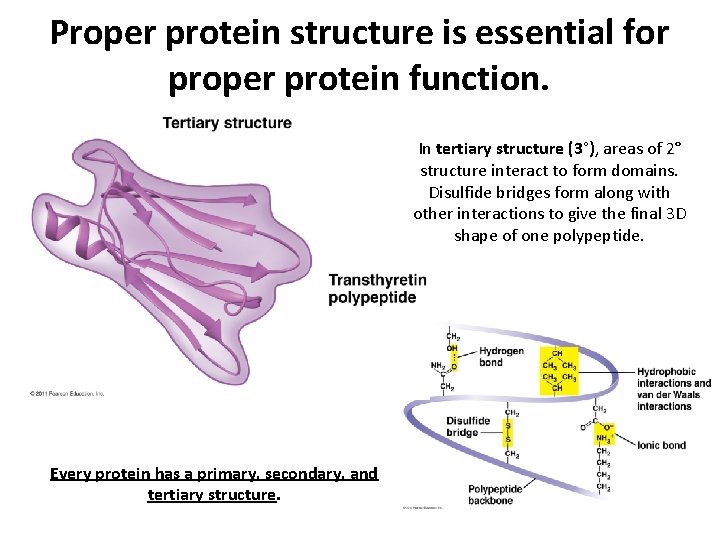
Proper protein structure is essential for proper protein function. In tertiary structure (3°), areas of 2° structure interact to form domains. Disulfide bridges form along with other interactions to give the final 3 D shape of one polypeptide. Every protein has a primary, secondary, and tertiary structure.
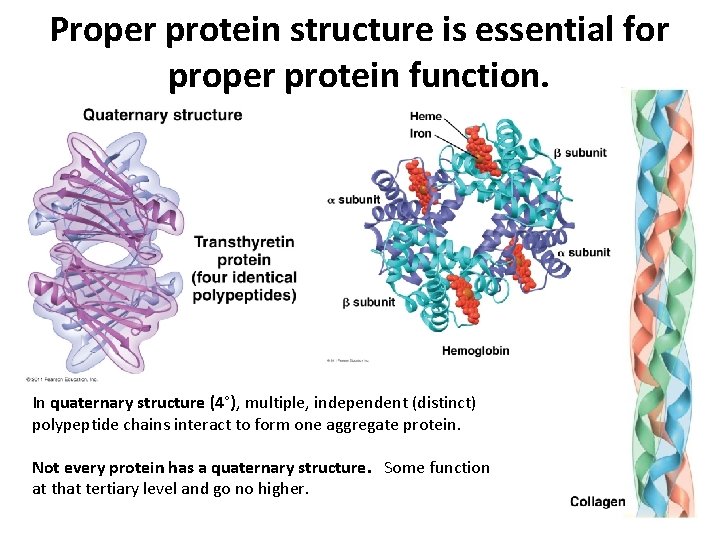
Proper protein structure is essential for proper protein function. In quaternary structure (4°), multiple, independent (distinct) polypeptide chains interact to form one aggregate protein. Not every protein has a quaternary structure. Some function at that tertiary level and go no higher.
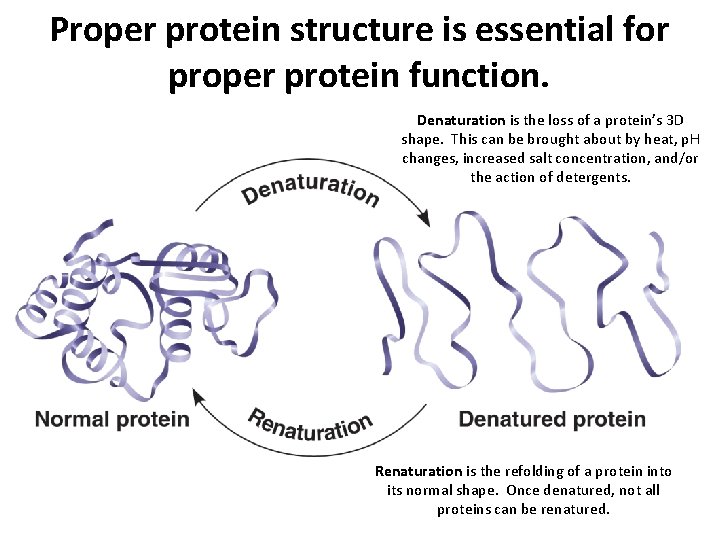
Proper protein structure is essential for proper protein function. Denaturation is the loss of a protein’s 3 D shape. This can be brought about by heat, p. H changes, increased salt concentration, and/or the action of detergents. Renaturation is the refolding of a protein into its normal shape. Once denatured, not all proteins can be renatured.
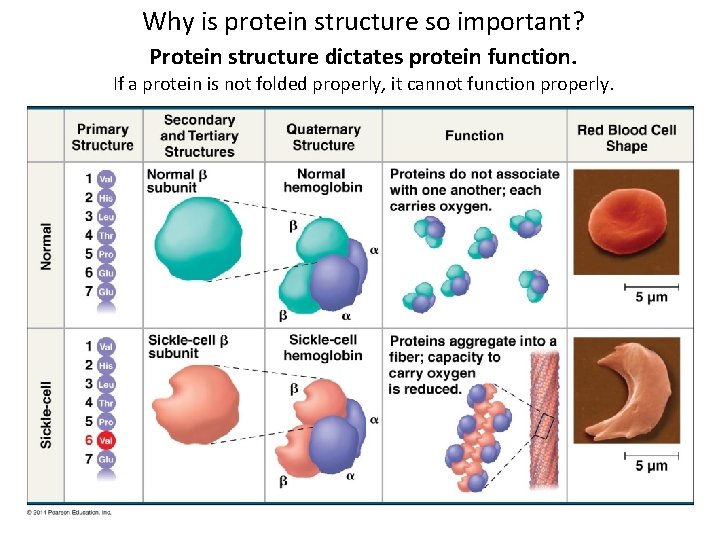
Why is protein structure so important? Protein structure dictates protein function. If a protein is not folded properly, it cannot function properly.
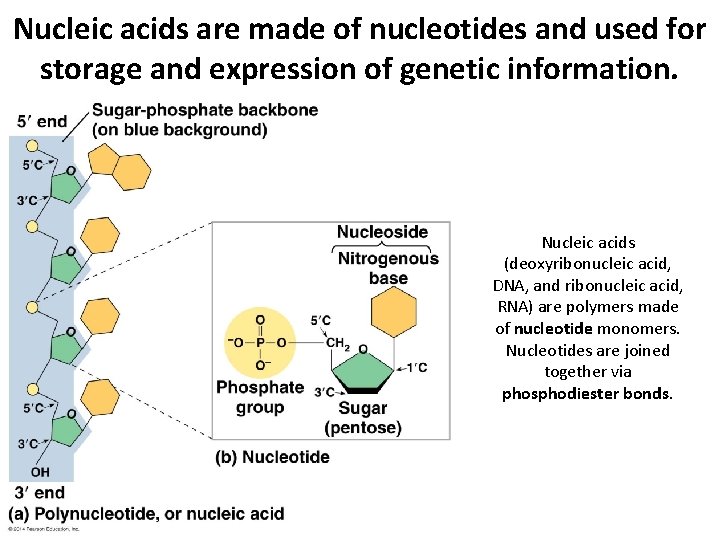
Nucleic acids are made of nucleotides and used for storage and expression of genetic information. Nucleic acids (deoxyribonucleic acid, DNA, and ribonucleic acid, RNA) are polymers made of nucleotide monomers. Nucleotides are joined together via phosphodiester bonds.
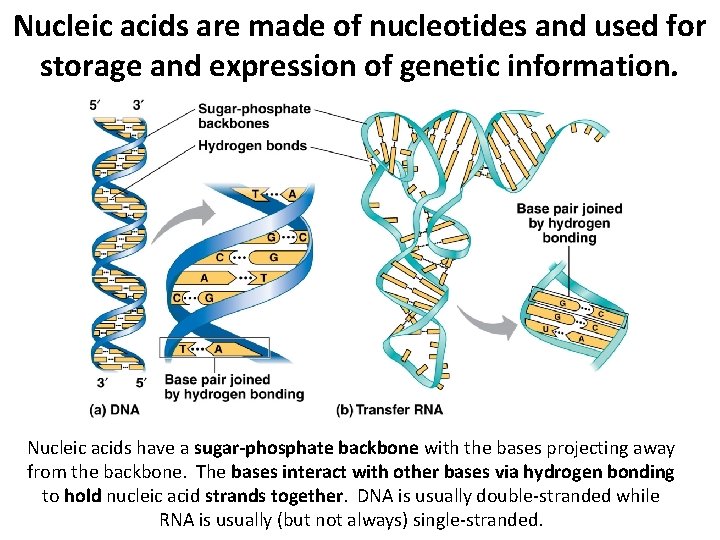
Nucleic acids are made of nucleotides and used for storage and expression of genetic information. Nucleic acids have a sugar-phosphate backbone with the bases projecting away from the backbone. The bases interact with other bases via hydrogen bonding to hold nucleic acid strands together. DNA is usually double-stranded while RNA is usually (but not always) single-stranded.
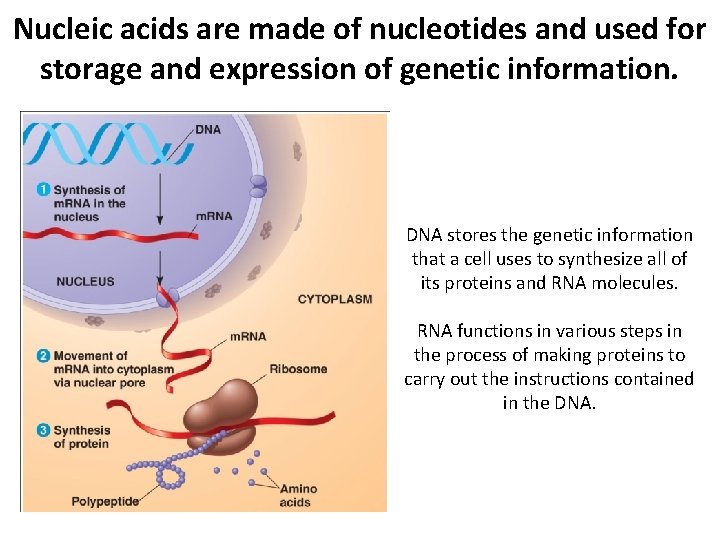
Nucleic acids are made of nucleotides and used for storage and expression of genetic information. DNA stores the genetic information that a cell uses to synthesize all of its proteins and RNA molecules. RNA functions in various steps in the process of making proteins to carry out the instructions contained in the DNA.
- Slides: 30