All isotopes atomic 84 and larger are naturally




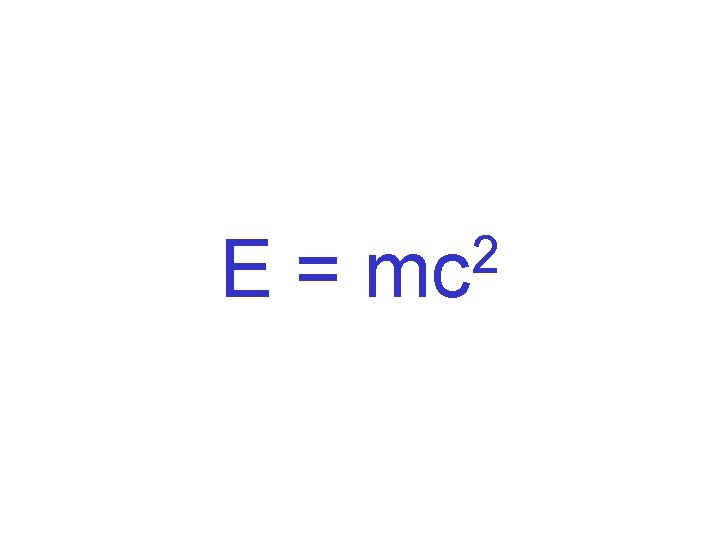



















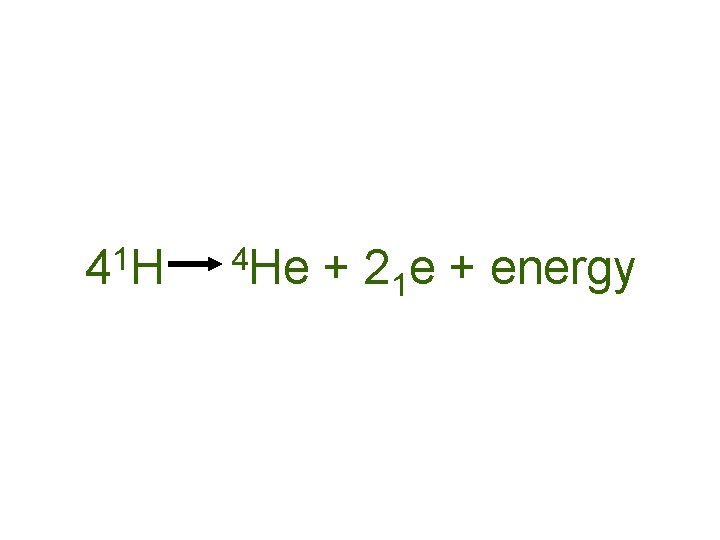











- Slides: 45


All isotopes atomic #84 and larger are naturally radioactive. Some isotopes smaller than atomic #84 are also naturally radioactive.

Artificial radioactivity can be produced by bombarding the nuclei of stable isotopes with high energy particles such as protons, neutrons, and alpha particles.

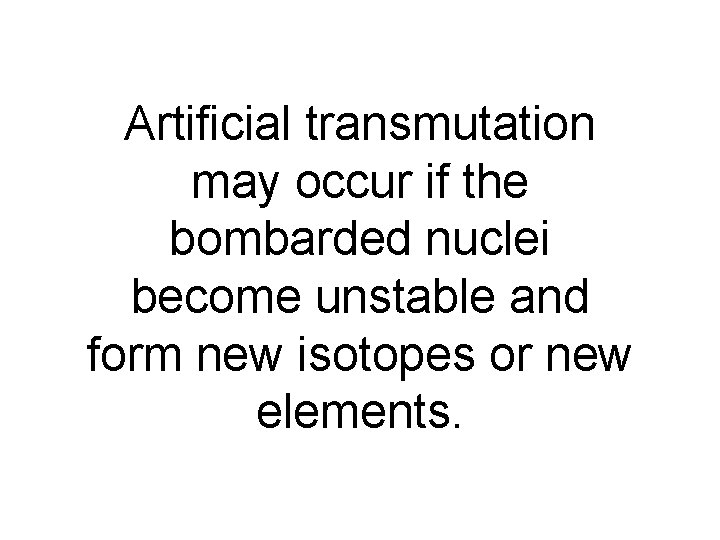
Artificial transmutation may occur if the bombarded nuclei become unstable and form new isotopes or new elements.

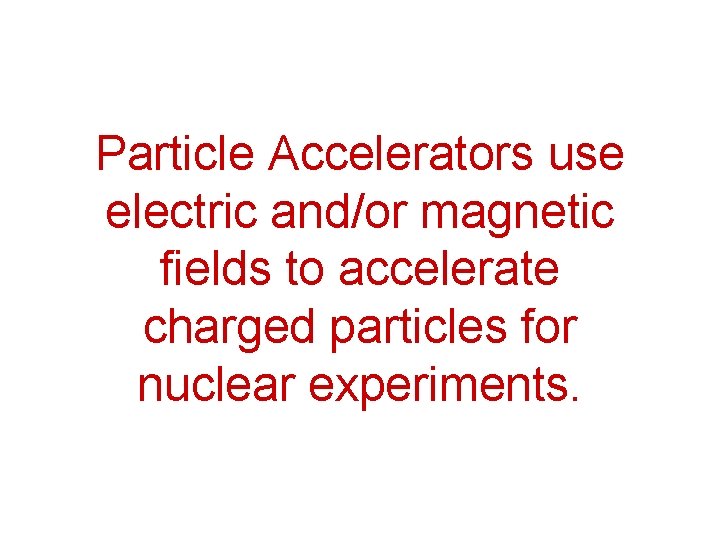
Particle Accelerators use electric and/or magnetic fields to accelerate charged particles for nuclear experiments.

Neutrons can NOT be accelerated by a particle accelerator. Why?

Energy is released in nuclear reactions because mass is converted into energy.
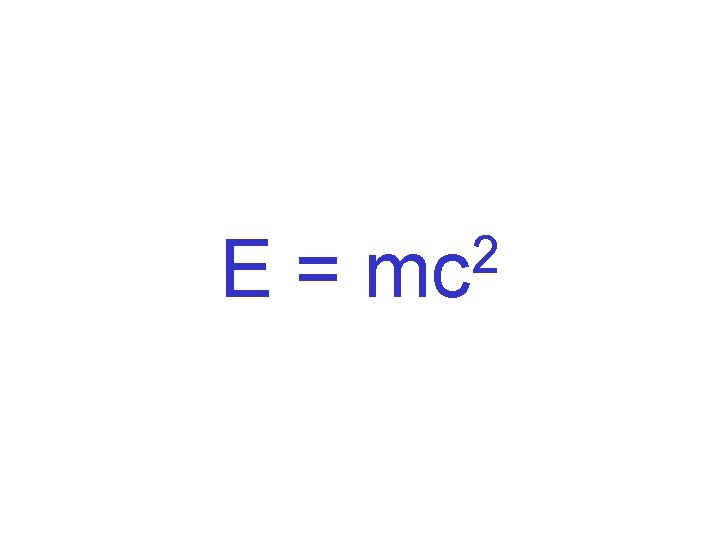
E= 2 mc

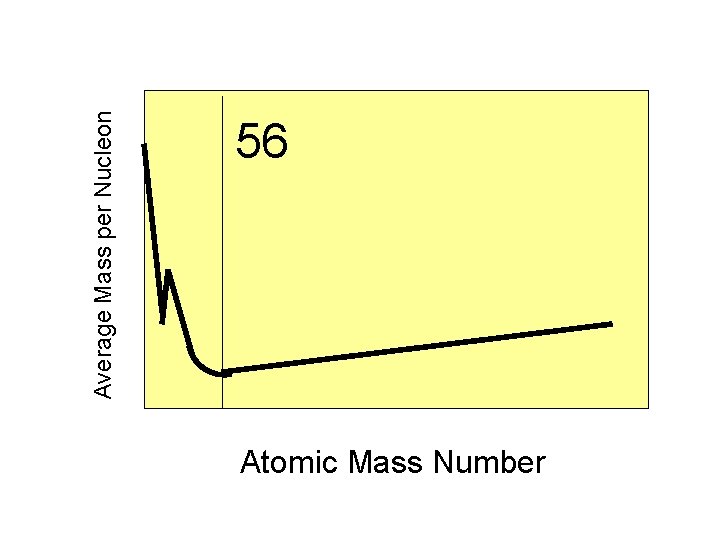
Average Mass per Nucleon 56 Atomic Mass Number

Protons and neutrons in the nucleus of iron-56 have the least amount of mass per nucleon of any element.

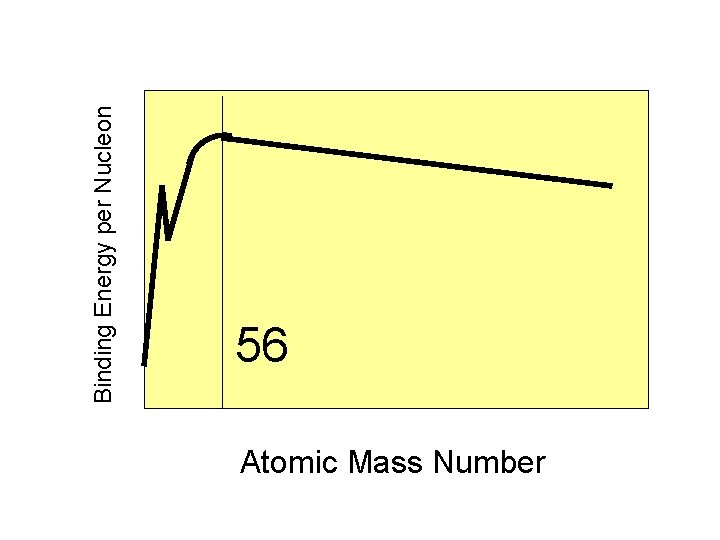
Binding Energy per Nucleon 56 Atomic Mass Number

Protons and neutrons in the nucleus of iron-56 have the highest binding energy per nucleon of any element.

Because of this, there are 2 general ways to produce nuclear energy!

Atoms larger than iron can be split to form 2 smaller atoms closer in size to iron-56. Splitting atoms is called nuclear fission.

Atoms smaller than iron can be joined to form a larger atom closer in size to iron-56. Joining atoms is called nuclear fusion.

Uranium-235 and plutonium-239 are currently used to carry out fission reactions in both nuclear weapons and nuclear power plants.

Neutrons are used to split the uranium-235 or plutonium-239 nuclei, producing energy, smaller isotopes, and more neutrons.

Producing more neutrons will cause more nuclei to be split, causing a “chain reaction”.

In an Atomic Bomb, this “chain reaction” is not controlled. A huge amount of energy is released almost instantly.

In a Nuclear Power Plant, the “chain reaction” is controlled. Here’s how this is done:

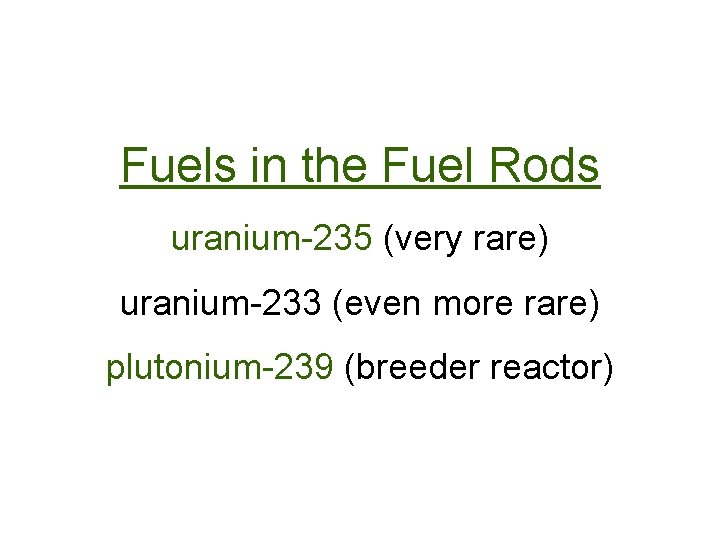
Fuels in the Fuel Rods uranium-235 (very rare) uranium-233 (even more rare) plutonium-239 (breeder reactor)

Neutrons must be slowed down to split the fuel. This is done by a “moderator”. (hydrogen, deuterium, beryllium, graphite or molten metals)

The rate of the reaction is controlled by “control rods” that absorb neutrons. (cadmium or boron)

The temperature is kept at reasonable levels by “coolants”. (water commonly used)

Protection from the radioactive materials is provided by “shielding”. (Steel protects the reactor while high density concrete protects the workers)

A “Breeder Reactor” uses U-235 for fuel while turning U-238 into Pu-239 which can also be used for fuel at a later time.

Splitting Uranium-235 nuclei produces over 200 different isotopes of 35 smaller elements. Some of these isotopes are radioactive.

The storage and disposal of these radioactive waste products is the main problem associated with generating electricity using nuclear fission.

Nuclear Fusion releases a much greater amount of energy than nuclear fission. Why?

Nuclear Fusion uses hydrogen (H-1) and deuterium (H-2) for fuel. Both are non-radioactive.
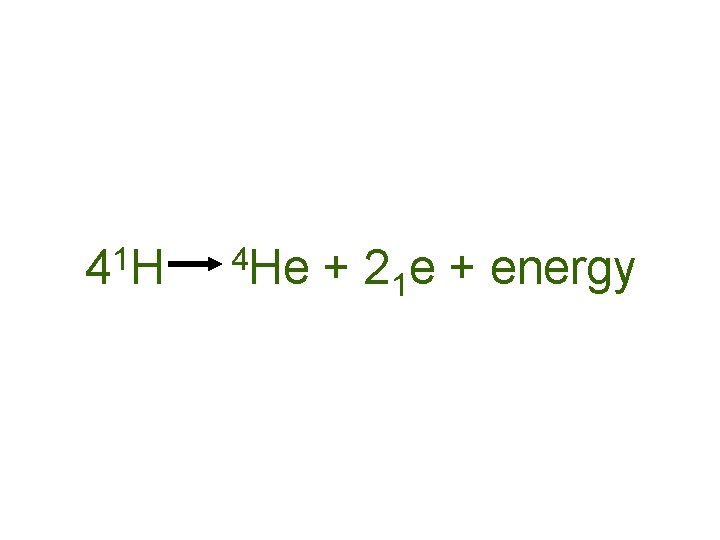
1 4 H 4 He + 21 e + energy

In addition to releasing more energy, the “waste product”, helium is nonradioactive and chemically stable.

So, if nuclear fusion is so much better than nuclear fission, why don’t we use it to generate electricity?

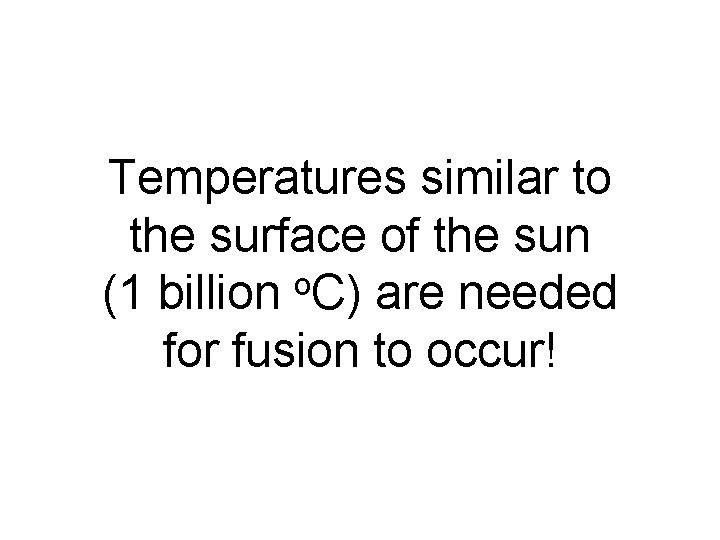
It takes extremely large amounts of energy to join two positively charged nuclei together.

Temperatures similar to the surface of the sun o (1 billion C) are needed for fusion to occur!

A hydrogen bomb (fusion) needs a small atomic bomb (fission) to start the fusion reaction!

There are several important uses of radioisotopes. One is the use of radioisotopes as tracers in chemical reactions.

Carbon-14 is often used to study organic chemical reactions like photosynthesis and respiration.

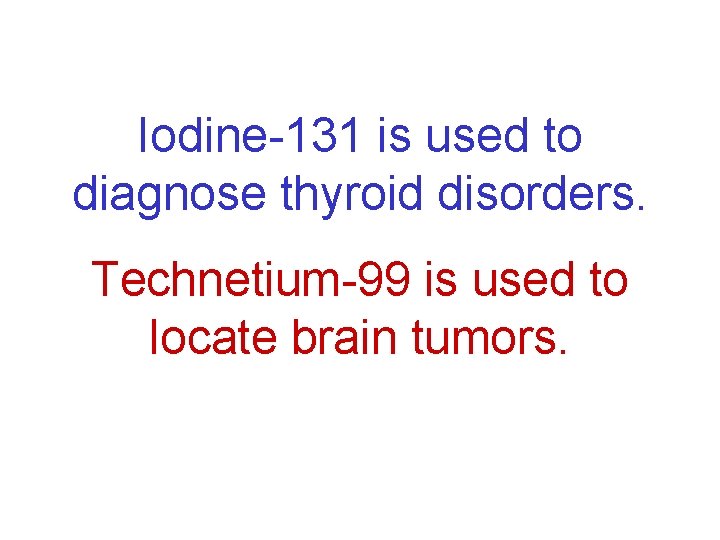
Iodine-131 is used to diagnose thyroid disorders. Technetium-99 is used to locate brain tumors.

Radium and cobalt-60 are sometimes used to kill cancer cells in a tumor.

Foods can be irradiated to kill bacteria, yeasts, and mold, to drastically improve shelf-life.

Radioisotopes are also useful for dating very old materials.

Carbon-14 can be used to determine the approximate age of once living things.

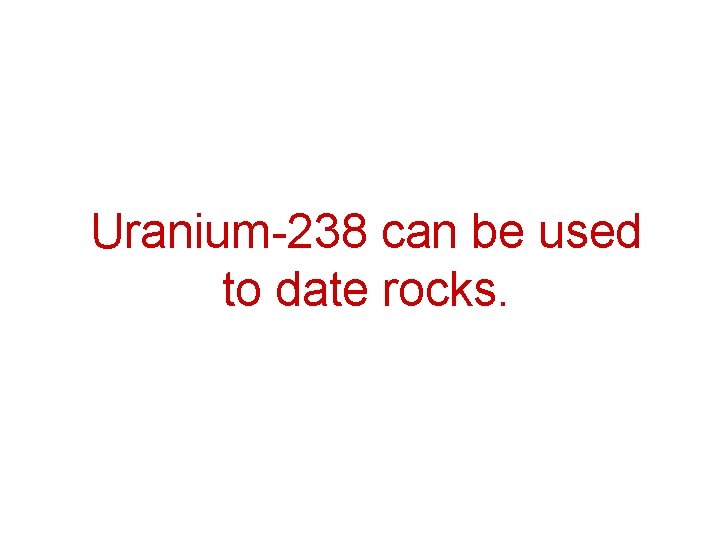
Uranium-238 can be used to date rocks.
