Alkyne and Reactions Nomenclature of alkynes Synthesis of


- Slides: 51

Alkyne and Reactions • Nomenclature of alkynes • Synthesis of alkyne through elimination rxn • Reactions of alkyne for regioselective synthesis of ketone/aldehyde • Reactions of alkyne for stereospecific synthesis of alkenes • Alkylation of alkyne for synthesis of elongated hydrocarbons • Halogenation and Ozonolysis of alkyne 10 -1

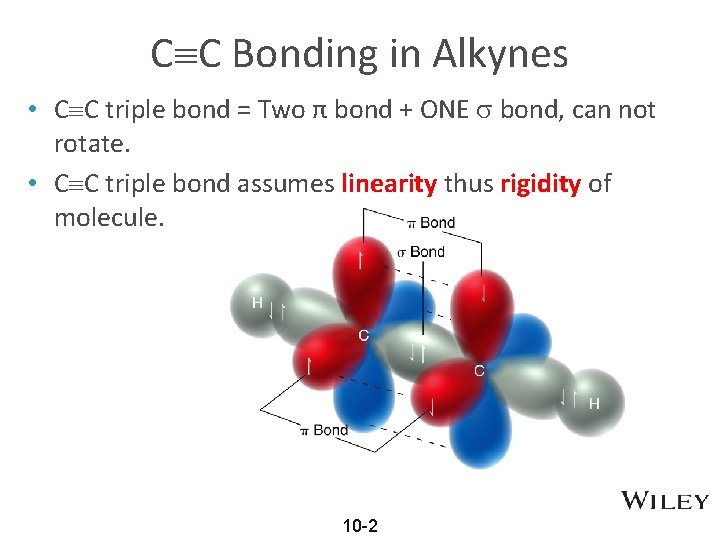
C C Bonding in Alkynes • C C triple bond = Two π bond + ONE bond, can not rotate. • C C triple bond assumes linearity thus rigidity of molecule. 10 -2

Alkynes: Nucleophile • The presence of two pi bonds and their associated electron density, alkynes are similar to alkenes as a nucleophile (albeit poor) • Converting pi bonds to sigma bonds generally makes a molecule more stable. 10 -3

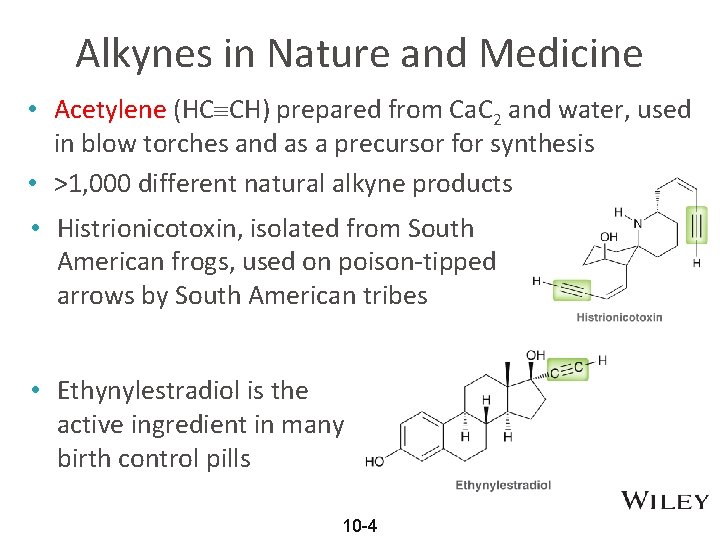
Alkynes in Nature and Medicine • Acetylene (HC CH) prepared from Ca. C 2 and water, used in blow torches and as a precursor for synthesis • >1, 000 different natural alkyne products • Histrionicotoxin, isolated from South American frogs, used on poison-tipped arrows by South American tribes • Ethynylestradiol is the active ingredient in many birth control pills 10 -4

1. Alkyne Nomenclature • Alkynes are named using the same procedure we used to name alkenes without E/Z or cis/trans 1. Identify the parent chain, which should include the C C triple bond 2. Identify and Name the substituents 3. Assign a locant (and prefix if necessary) to each substituent giving the C C triple bond the lowest number possible 4. List the numbered substituents before the parent name in alphabetical order. Ignore prefixes (except iso) when ordering alphabetically 5. The C C triple bond locant is placed either just before the parent name or just before the -yne suffix 10 -5

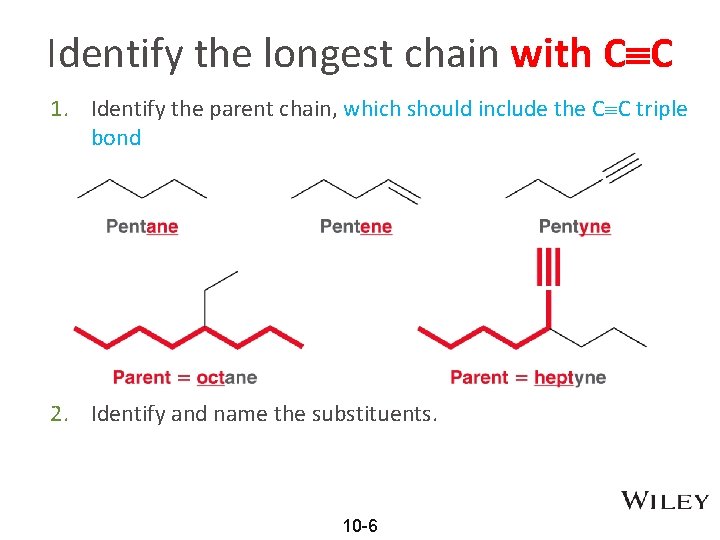
Identify the longest chain with C C 1. Identify the parent chain, which should include the C C triple bond 2. Identify and name the substituents. 10 -6

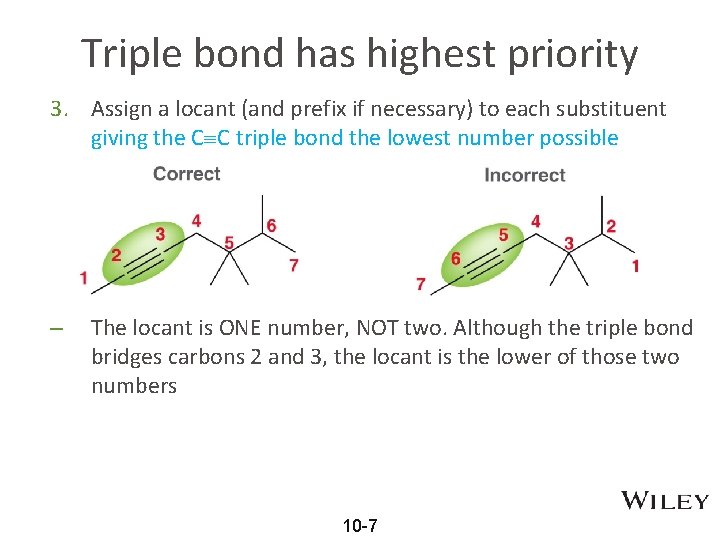
Triple bond has highest priority 3. Assign a locant (and prefix if necessary) to each substituent giving the C C triple bond the lowest number possible – The locant is ONE number, NOT two. Although the triple bond bridges carbons 2 and 3, the locant is the lower of those two numbers 10 -7

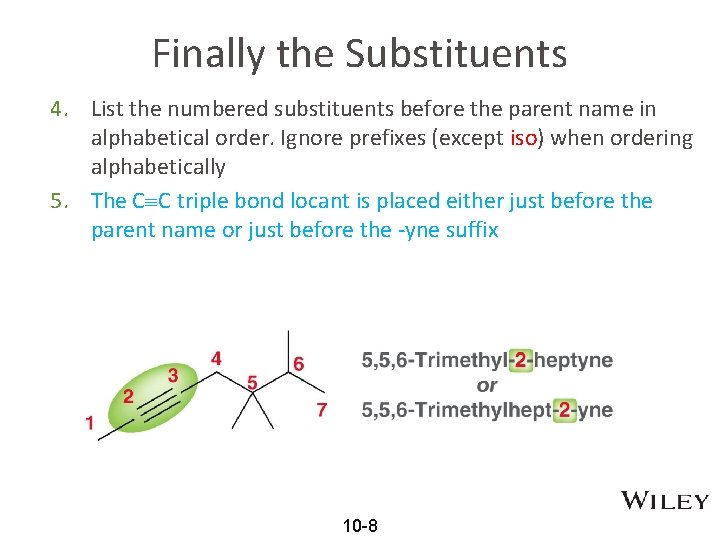
Finally the Substituents 4. List the numbered substituents before the parent name in alphabetical order. Ignore prefixes (except iso) when ordering alphabetically 5. The C C triple bond locant is placed either just before the parent name or just before the -yne suffix 10 -8

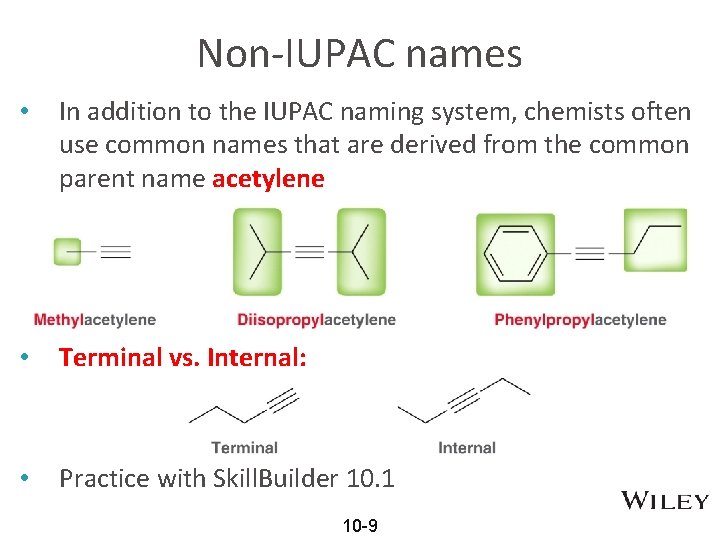
Non-IUPAC names • In addition to the IUPAC naming system, chemists often use common names that are derived from the common parent name acetylene • Terminal vs. Internal: • Practice with Skill. Builder 10. 1 10 -9

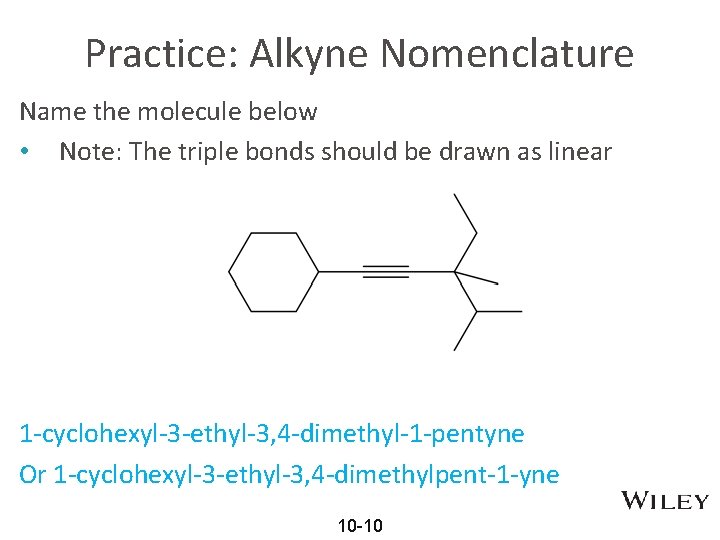
Practice: Alkyne Nomenclature Name the molecule below • Note: The triple bonds should be drawn as linear 1 -cyclohexyl-3 -ethyl-3, 4 -dimethyl-1 -pentyne Or 1 -cyclohexyl-3 -ethyl-3, 4 -dimethylpent-1 -yne 10 -10

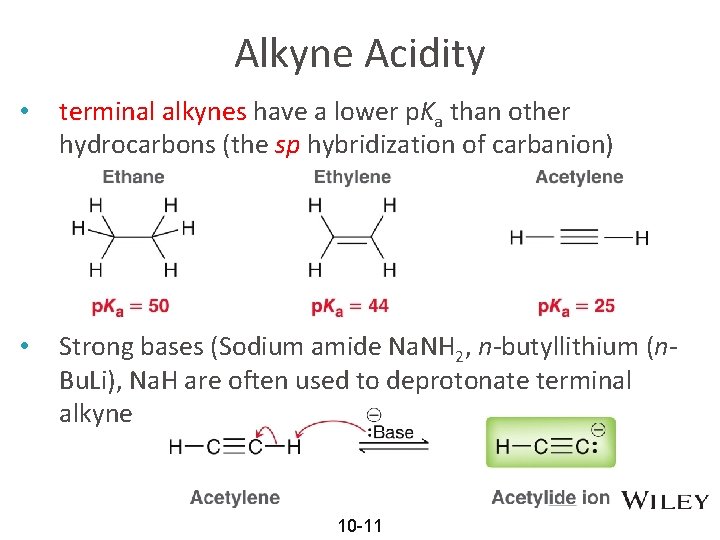
Alkyne Acidity • terminal alkynes have a lower p. Ka than other hydrocarbons (the sp hybridization of carbanion) • Strong bases (Sodium amide Na. NH 2, n-butyllithium (n. Bu. Li), Na. H are often used to deprotonate terminal alkyne 10 -11

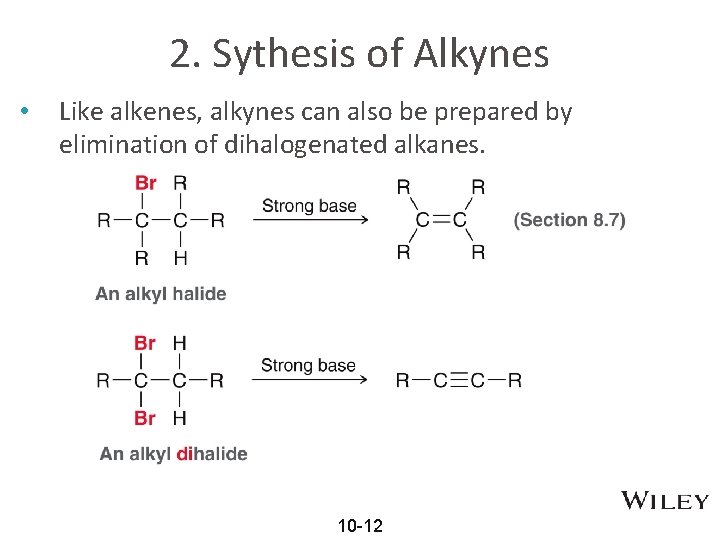
2. Sythesis of Alkynes • Like alkenes, alkynes can also be prepared by elimination of dihalogenated alkanes. 10 -12

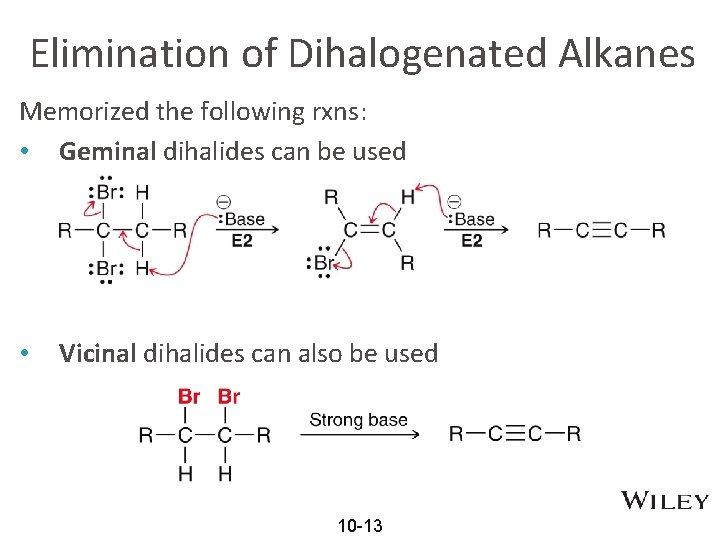
Elimination of Dihalogenated Alkanes Memorized the following rxns: • Geminal dihalides can be used • Vicinal dihalides can also be used 10 -13

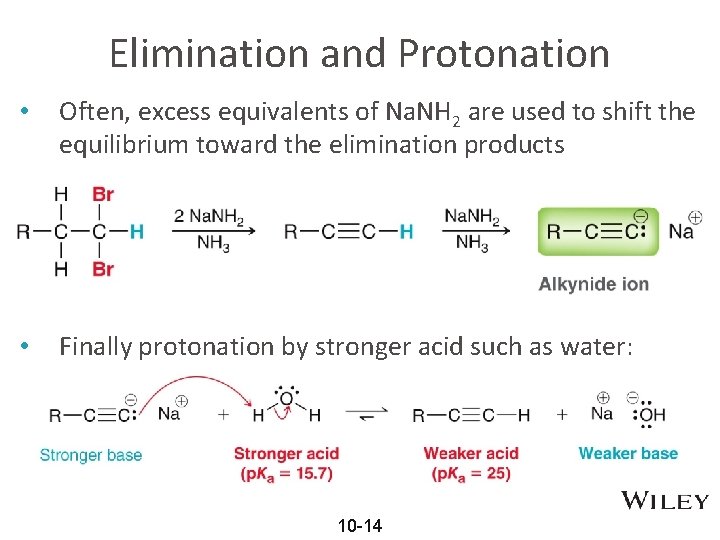
Elimination and Protonation • Often, excess equivalents of Na. NH 2 are used to shift the equilibrium toward the elimination products • Finally protonation by stronger acid such as water: 10 -14

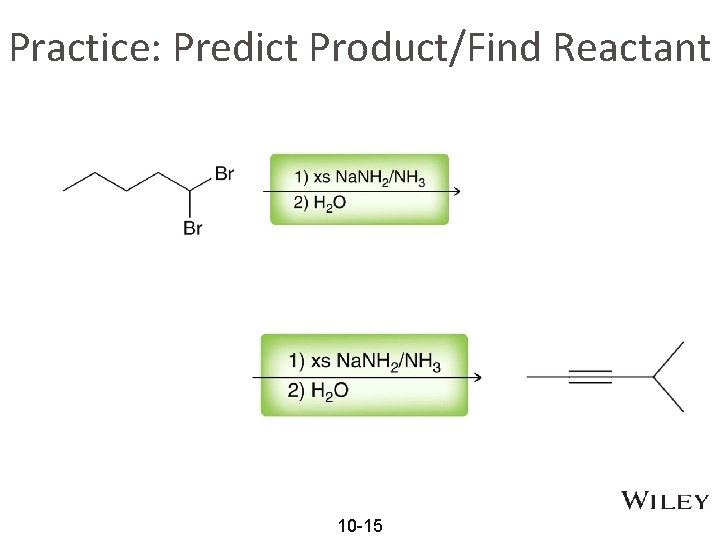
Practice: Predict Product/Find Reactant 10 -15

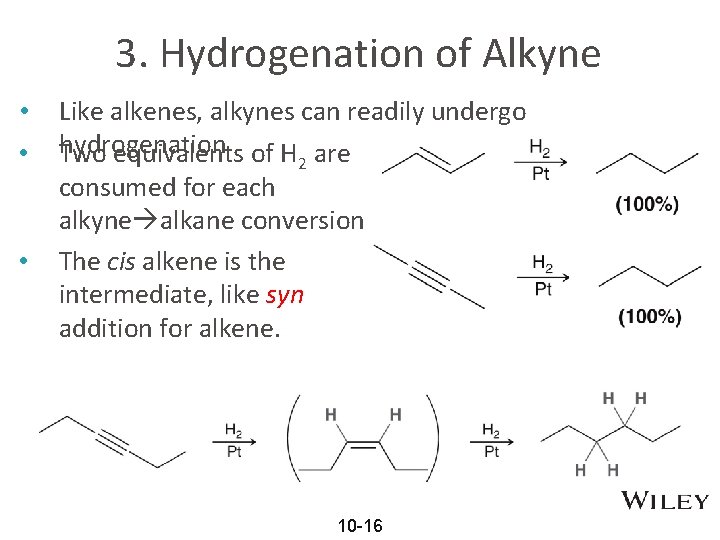
3. Hydrogenation of Alkyne • • • Like alkenes, alkynes can readily undergo hydrogenation Two equivalents of H are 2 consumed for each alkyne alkane conversion The cis alkene is the intermediate, like syn addition for alkene. 10 -16

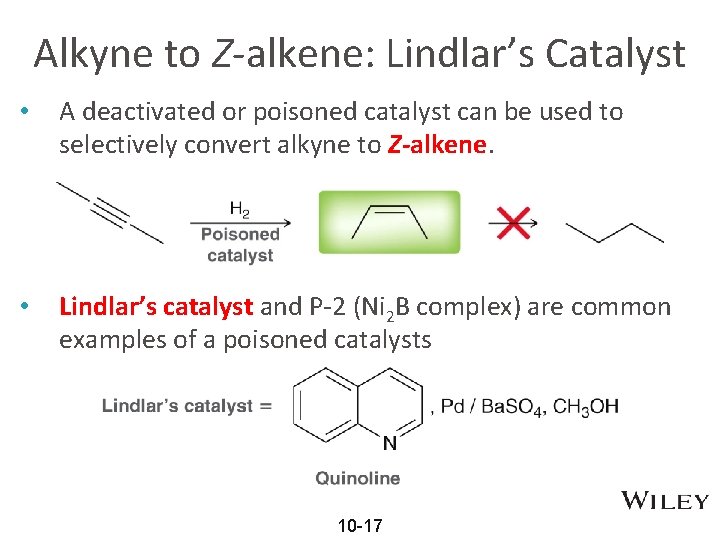
Alkyne to Z-alkene: Lindlar’s Catalyst • A deactivated or poisoned catalyst can be used to selectively convert alkyne to Z-alkene. • Lindlar’s catalyst and P-2 (Ni 2 B complex) are common examples of a poisoned catalysts 10 -17

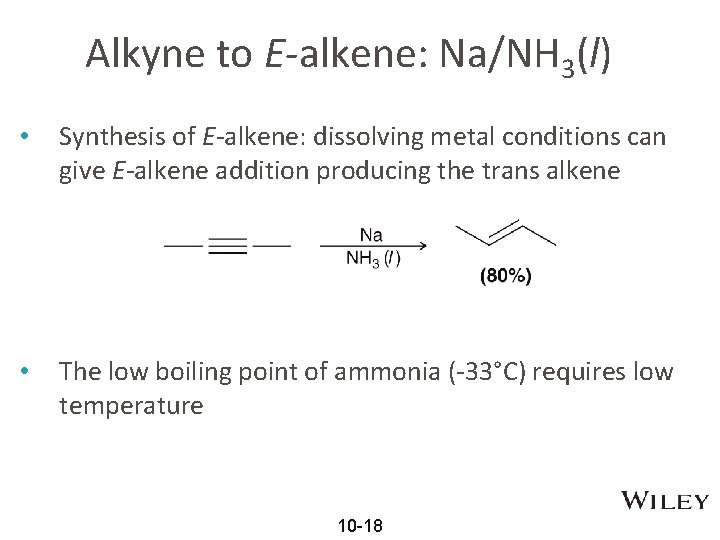
Alkyne to E-alkene: Na/NH 3(l) • Synthesis of E-alkene: dissolving metal conditions can give E-alkene addition producing the trans alkene • The low boiling point of ammonia (-33°C) requires low temperature 10 -18

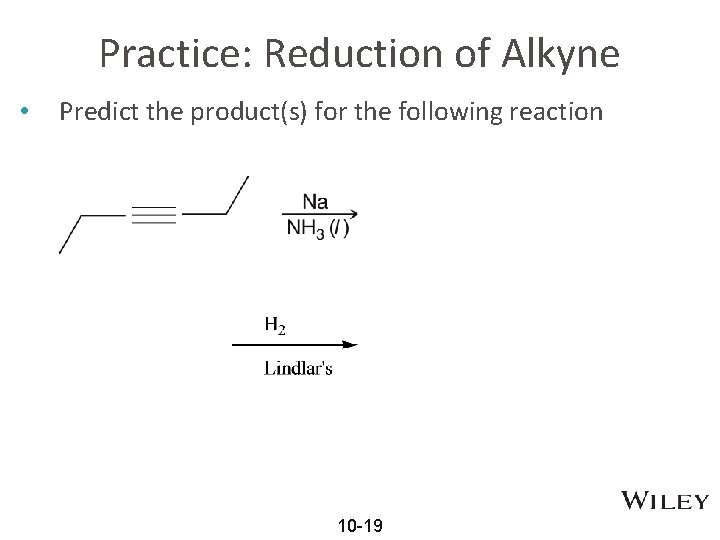
Practice: Reduction of Alkyne • Predict the product(s) for the following reaction 10 -19

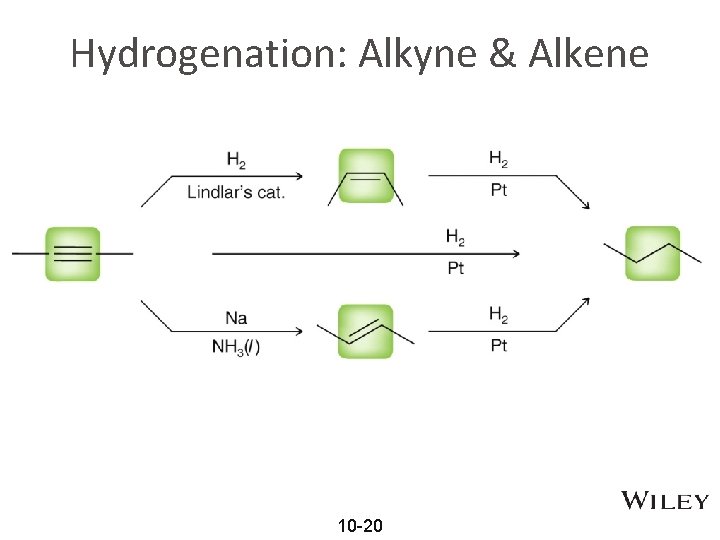
Hydrogenation: Alkyne & Alkene 10 -20

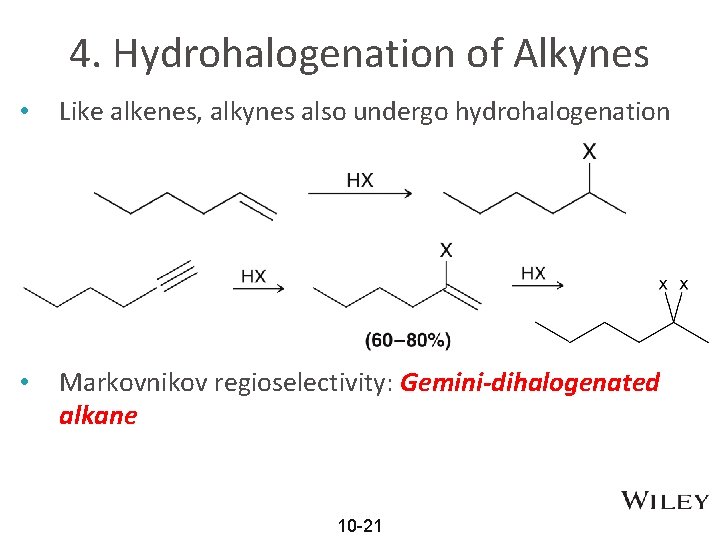
4. Hydrohalogenation of Alkynes • Like alkenes, alkynes also undergo hydrohalogenation • Markovnikov regioselectivity: Gemini-dihalogenated alkane 10 -21

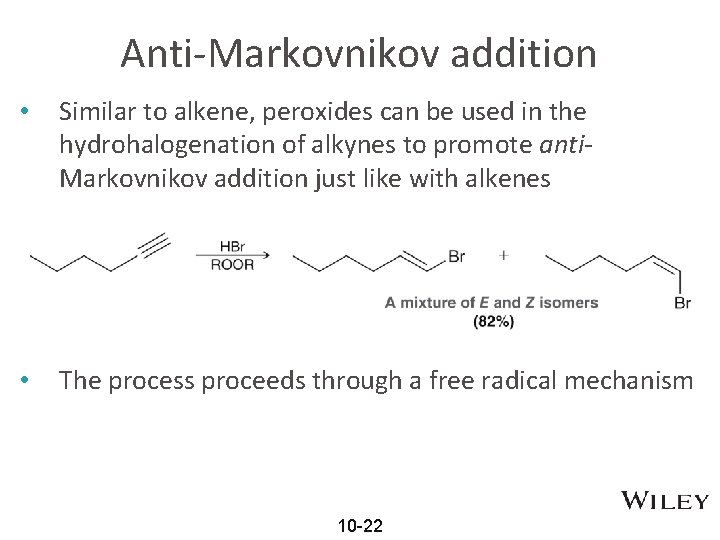
Anti-Markovnikov addition • Similar to alkene, peroxides can be used in the hydrohalogenation of alkynes to promote anti. Markovnikov addition just like with alkenes • The process proceeds through a free radical mechanism 10 -22

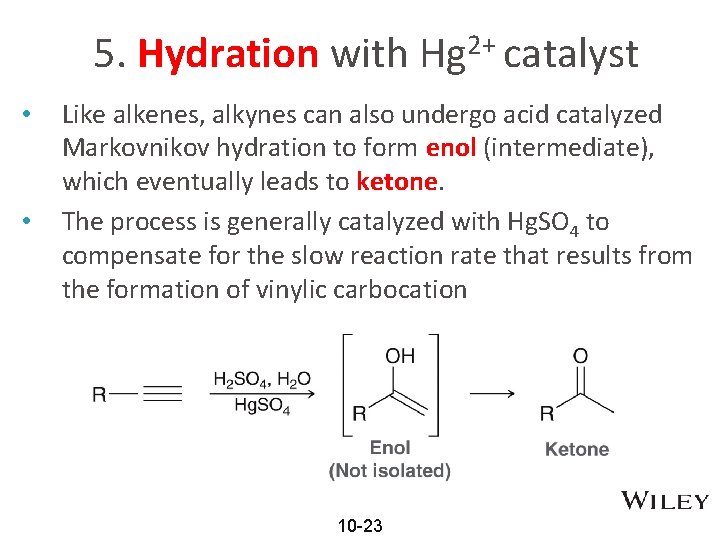
5. Hydration with Hg 2+ catalyst • • Like alkenes, alkynes can also undergo acid catalyzed Markovnikov hydration to form enol (intermediate), which eventually leads to ketone. The process is generally catalyzed with Hg. SO 4 to compensate for the slow reaction rate that results from the formation of vinylic carbocation 10 -23

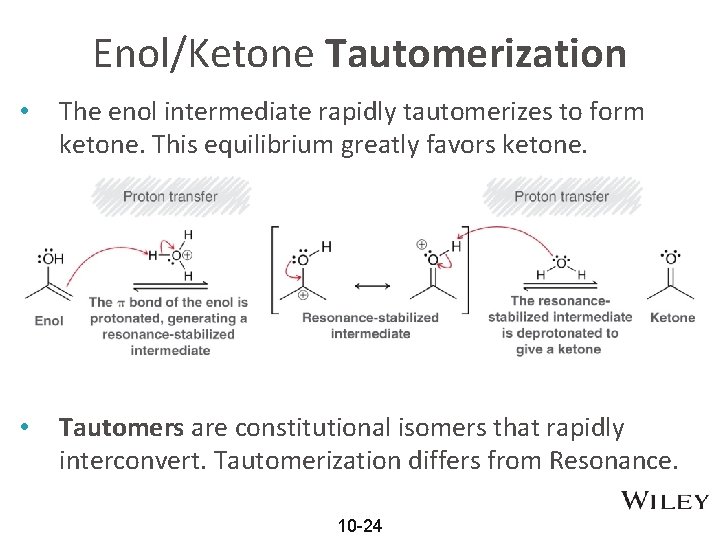
Enol/Ketone Tautomerization • The enol intermediate rapidly tautomerizes to form ketone. This equilibrium greatly favors ketone. • Tautomers are constitutional isomers that rapidly interconvert. Tautomerization differs from Resonance. 10 -24

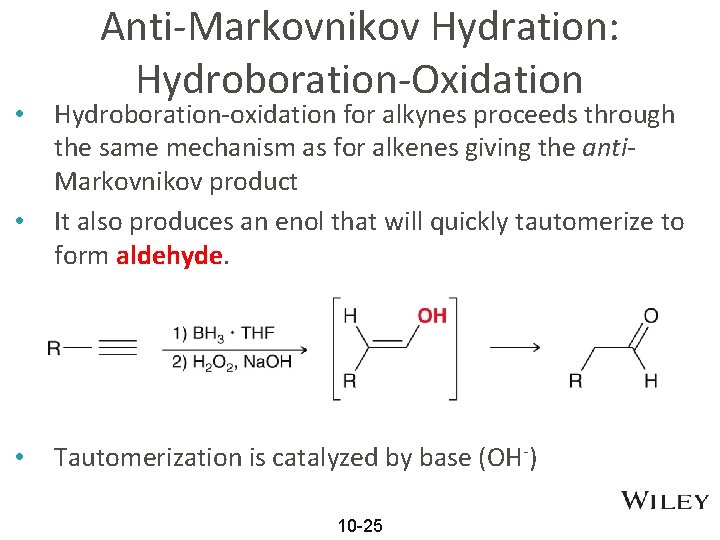
• • • Anti-Markovnikov Hydration: Hydroboration-Oxidation Hydroboration-oxidation for alkynes proceeds through the same mechanism as for alkenes giving the anti. Markovnikov product It also produces an enol that will quickly tautomerize to form aldehyde. Tautomerization is catalyzed by base (OH-) 10 -25

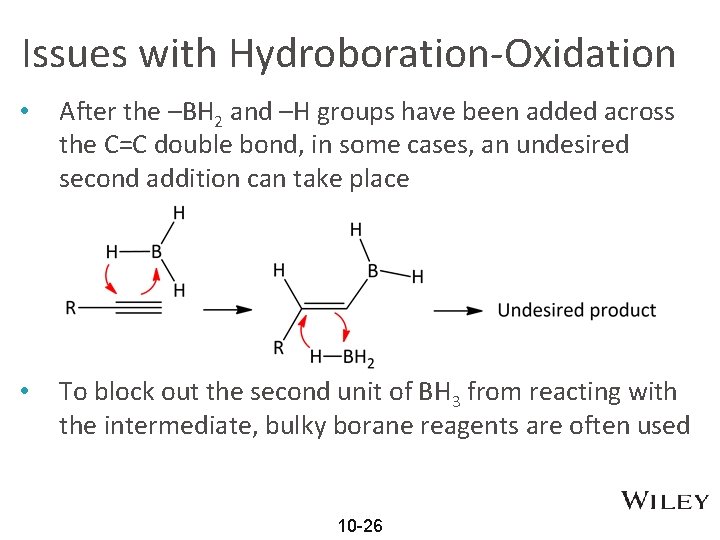
Issues with Hydroboration-Oxidation • After the –BH 2 and –H groups have been added across the C=C double bond, in some cases, an undesired second addition can take place • To block out the second unit of BH 3 from reacting with the intermediate, bulky borane reagents are often used 10 -26

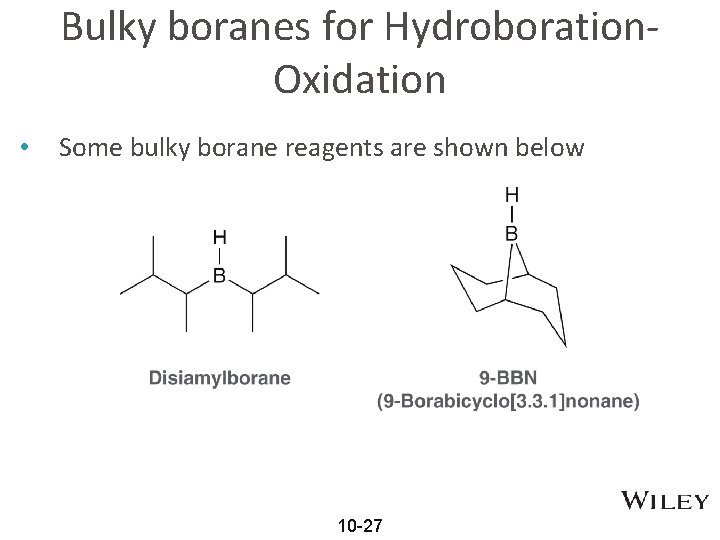
Bulky boranes for Hydroboration. Oxidation • Some bulky borane reagents are shown below 10 -27

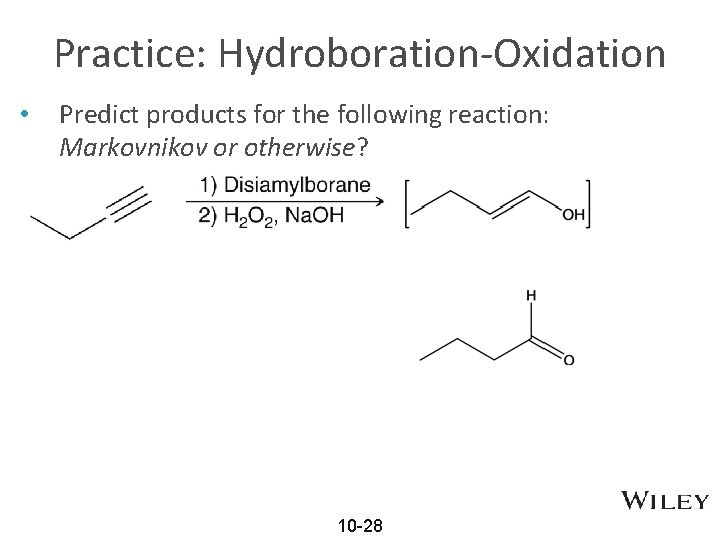
Practice: Hydroboration-Oxidation • Predict products for the following reaction: Markovnikov or otherwise? 10 -28

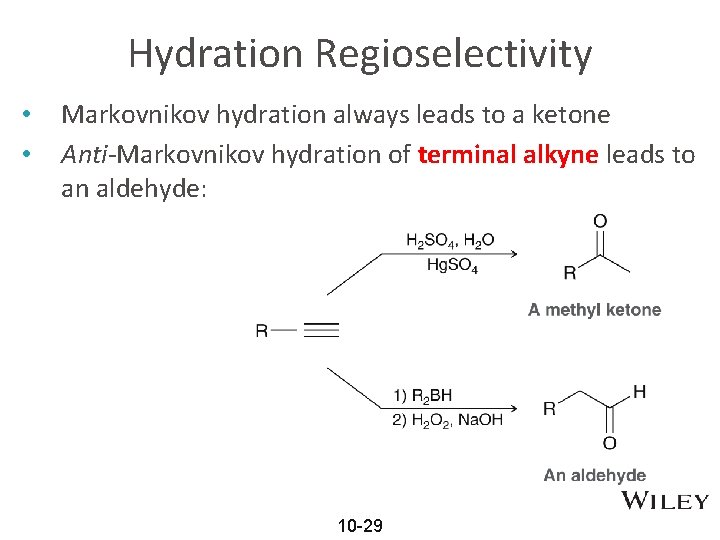
Hydration Regioselectivity • • Markovnikov hydration always leads to a ketone Anti-Markovnikov hydration of terminal alkyne leads to an aldehyde: 10 -29

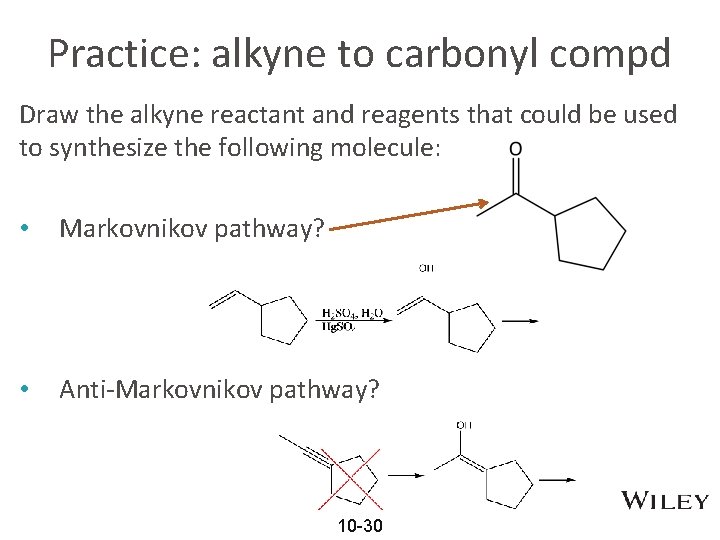
Practice: alkyne to carbonyl compd Draw the alkyne reactant and reagents that could be used to synthesize the following molecule: • Markovnikov pathway? • Anti-Markovnikov pathway? 10 -30

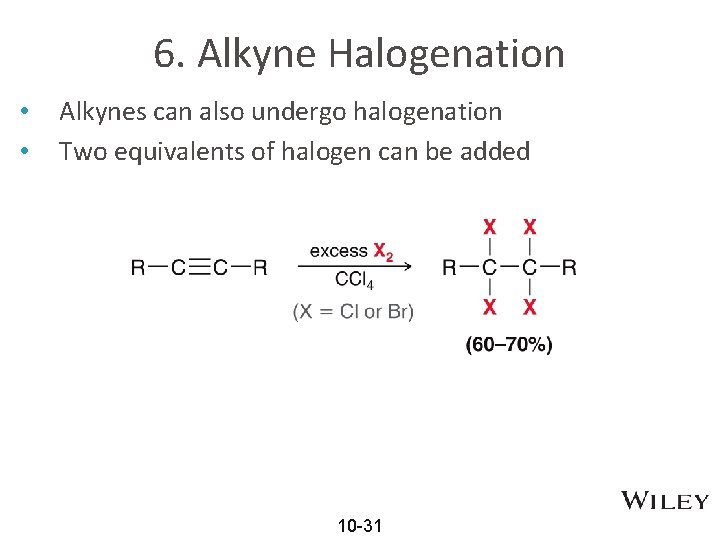
6. Alkyne Halogenation • • Alkynes can also undergo halogenation Two equivalents of halogen can be added 10 -31

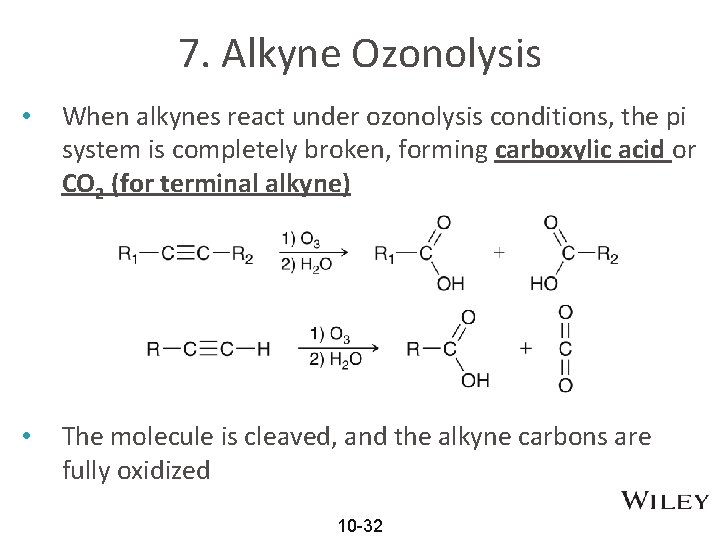
7. Alkyne Ozonolysis • When alkynes react under ozonolysis conditions, the pi system is completely broken, forming carboxylic acid or CO 2 (for terminal alkyne) • The molecule is cleaved, and the alkyne carbons are fully oxidized 10 -32

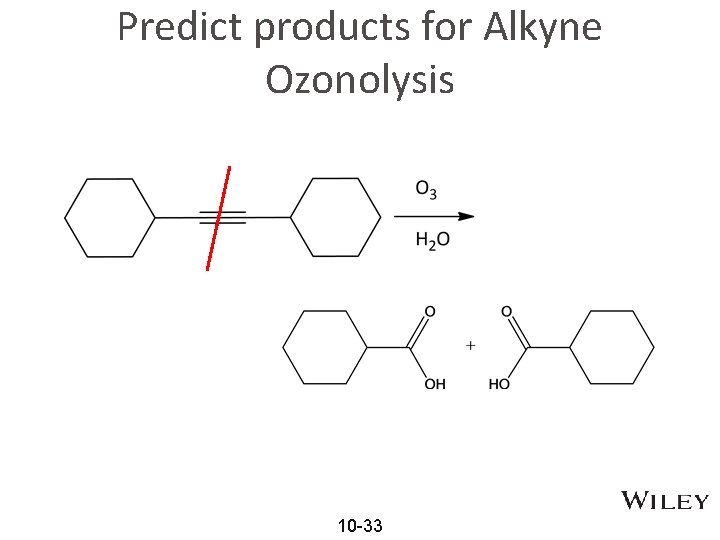
Predict products for Alkyne Ozonolysis 10 -33

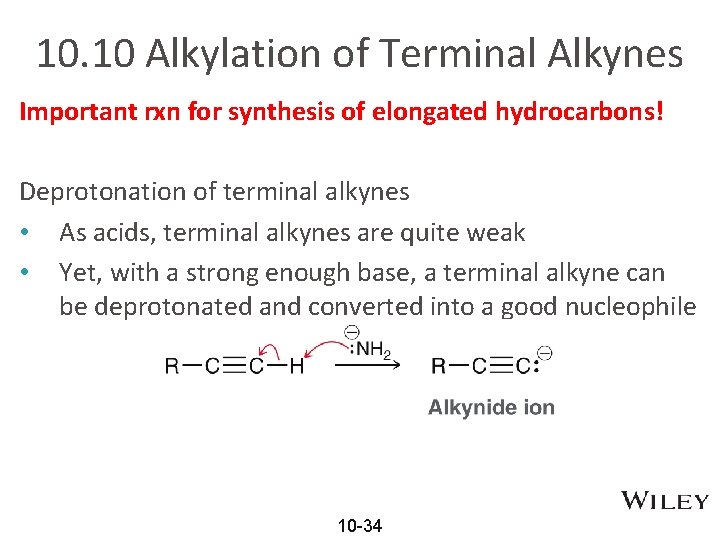
10. 10 Alkylation of Terminal Alkynes Important rxn for synthesis of elongated hydrocarbons! Deprotonation of terminal alkynes • As acids, terminal alkynes are quite weak • Yet, with a strong enough base, a terminal alkyne can be deprotonated and converted into a good nucleophile 10 -34

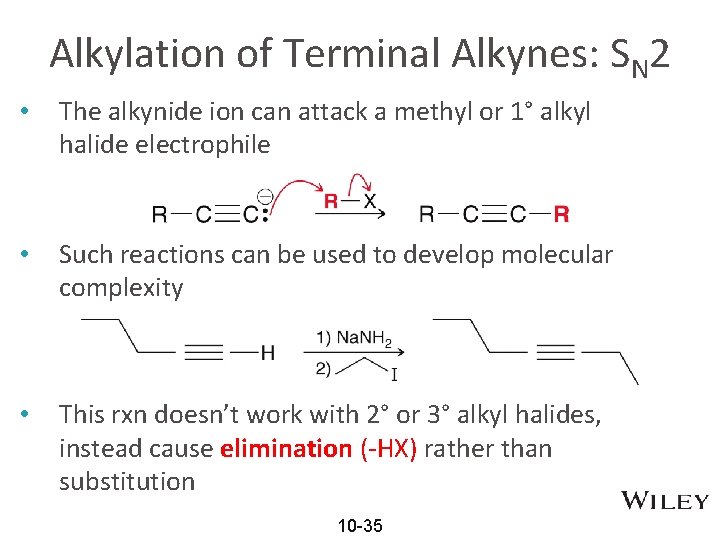
Alkylation of Terminal Alkynes: SN 2 • The alkynide ion can attack a methyl or 1° alkyl halide electrophile • Such reactions can be used to develop molecular complexity • This rxn doesn’t work with 2° or 3° alkyl halides, instead cause elimination (-HX) rather than substitution 10 -35

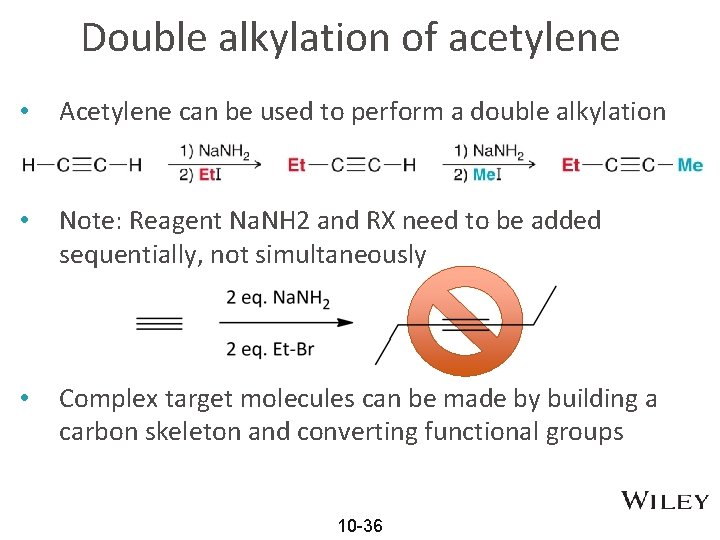
Double alkylation of acetylene • Acetylene can be used to perform a double alkylation • Note: Reagent Na. NH 2 and RX need to be added sequentially, not simultaneously • Complex target molecules can be made by building a carbon skeleton and converting functional groups 10 -36

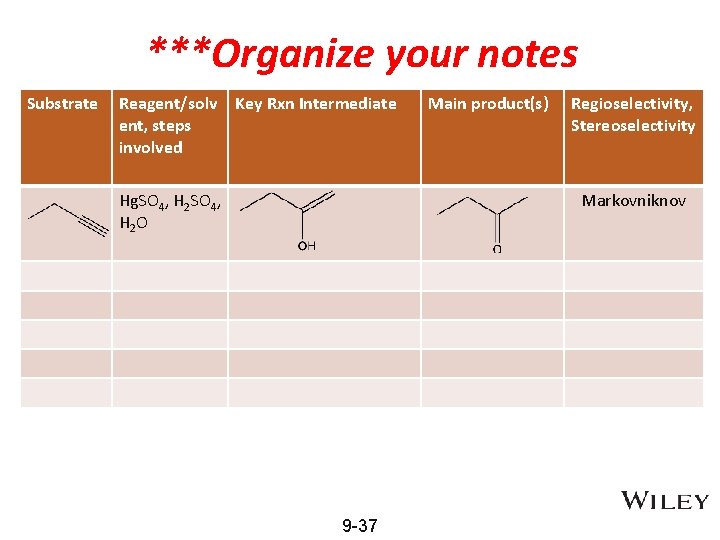
***Organize your notes Substrate Reagent/solv Key Rxn Intermediate ent, steps involved Hg. SO 4, H 2 O Main product(s) Regioselectivity, Stereoselectivity Markovniknov 9 -37

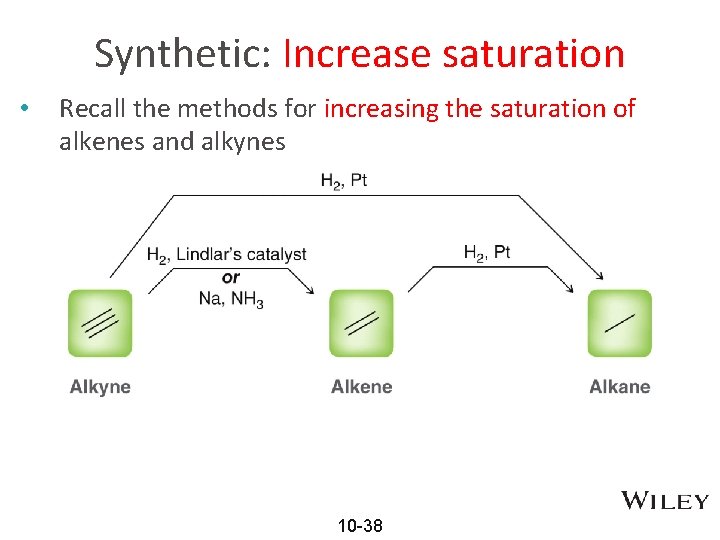
Synthetic: Increase saturation • Recall the methods for increasing the saturation of alkenes and alkynes 10 -38

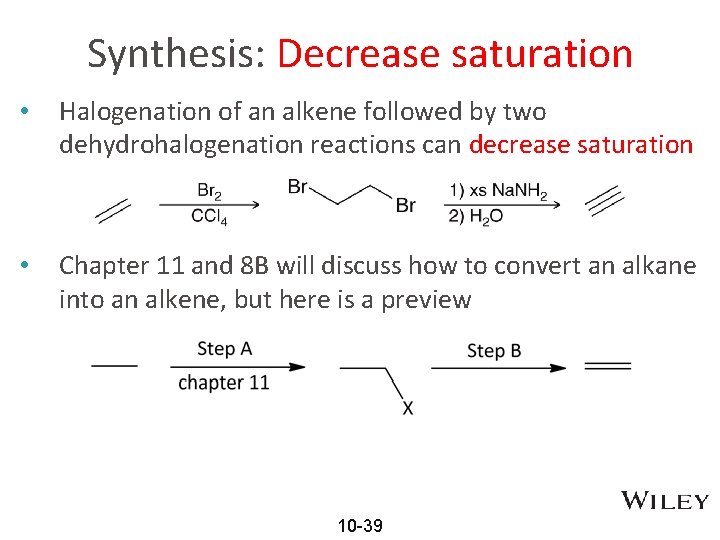
Synthesis: Decrease saturation • Halogenation of an alkene followed by two dehydrohalogenation reactions can decrease saturation • Chapter 11 and 8 B will discuss how to convert an alkane into an alkene, but here is a preview 10 -39

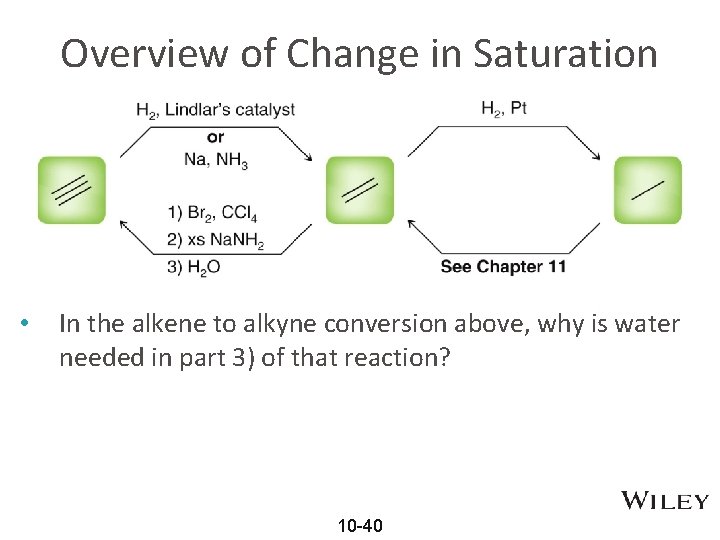
Overview of Change in Saturation • In the alkene to alkyne conversion above, why is water needed in part 3) of that reaction? 10 -40

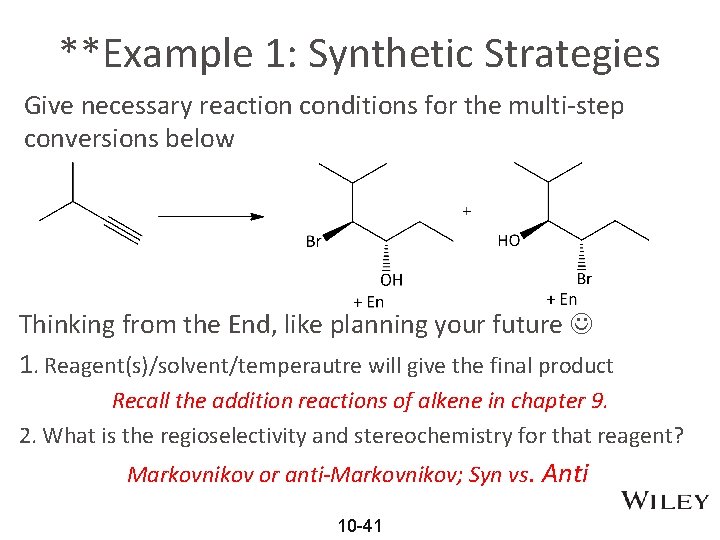
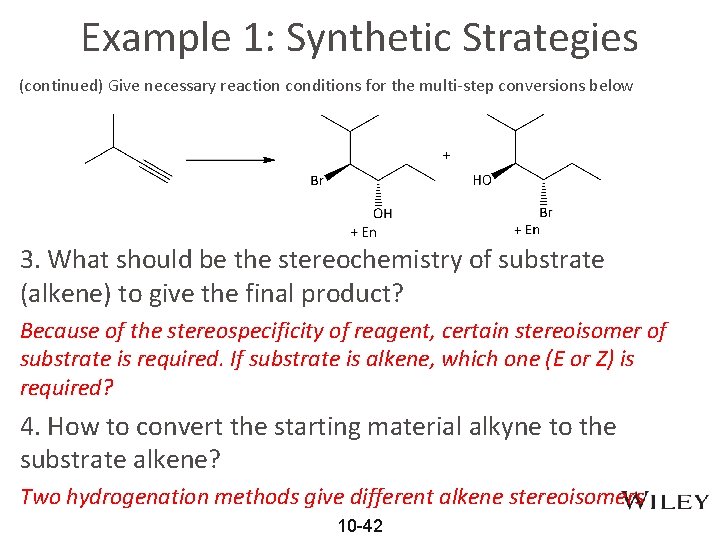
**Example 1: Synthetic Strategies Give necessary reaction conditions for the multi-step conversions below Thinking from the End, like planning your future 1. Reagent(s)/solvent/temperautre will give the final product Recall the addition reactions of alkene in chapter 9. 2. What is the regioselectivity and stereochemistry for that reagent? Markovnikov or anti-Markovnikov; Syn vs. Anti 10 -41

Example 1: Synthetic Strategies (continued) Give necessary reaction conditions for the multi-step conversions below 3. What should be the stereochemistry of substrate (alkene) to give the final product? Because of the stereospecificity of reagent, certain stereoisomer of substrate is required. If substrate is alkene, which one (E or Z) is required? 4. How to convert the starting material alkyne to the substrate alkene? Two hydrogenation methods give different alkene stereoisomers 10 -42

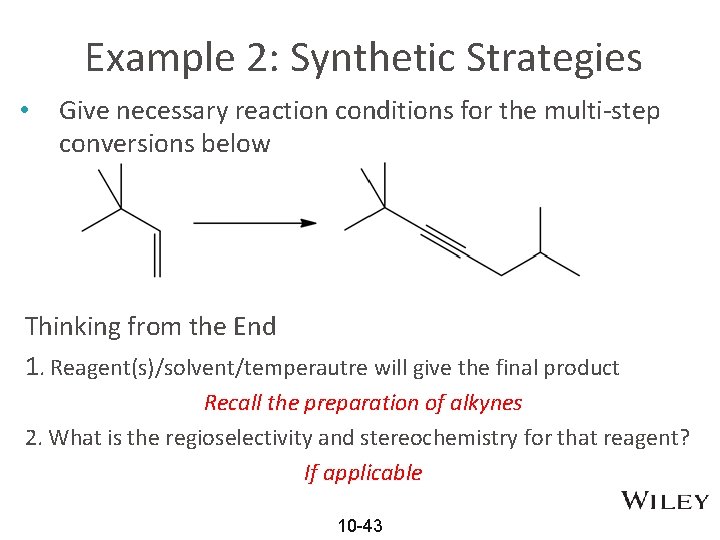
Example 2: Synthetic Strategies • Give necessary reaction conditions for the multi-step conversions below Thinking from the End 1. Reagent(s)/solvent/temperautre will give the final product Recall the preparation of alkynes 2. What is the regioselectivity and stereochemistry for that reagent? If applicable 10 -43

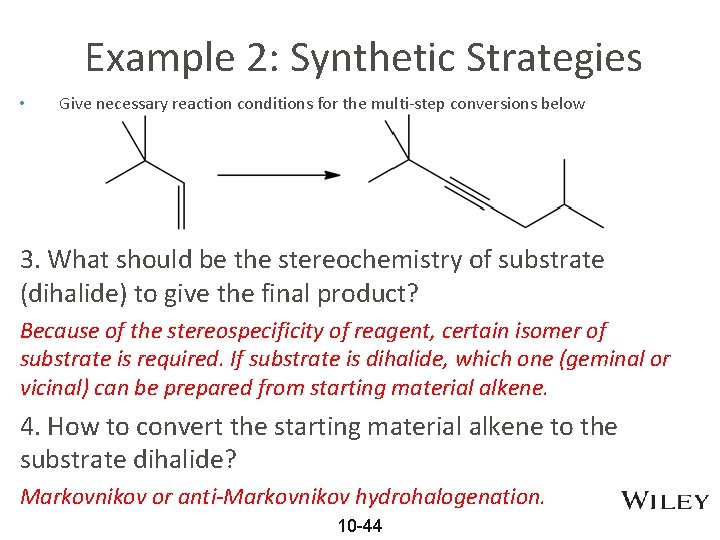
Example 2: Synthetic Strategies • Give necessary reaction conditions for the multi-step conversions below 3. What should be the stereochemistry of substrate (dihalide) to give the final product? Because of the stereospecificity of reagent, certain isomer of substrate is required. If substrate is dihalide, which one (geminal or vicinal) can be prepared from starting material alkene. 4. How to convert the starting material alkene to the substrate dihalide? Markovnikov or anti-Markovnikov hydrohalogenation. 10 -44

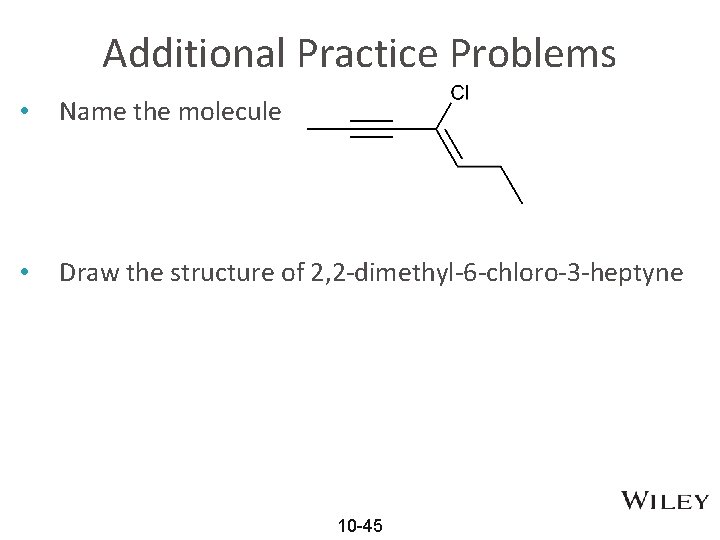
Additional Practice Problems • Name the molecule • Draw the structure of 2, 2 -dimethyl-6 -chloro-3 -heptyne 10 -45

Additional Practice Problems • Give 2 sets of reagents that could be used to synthesize 1 -pentyne through elimination reactions. 10 -46

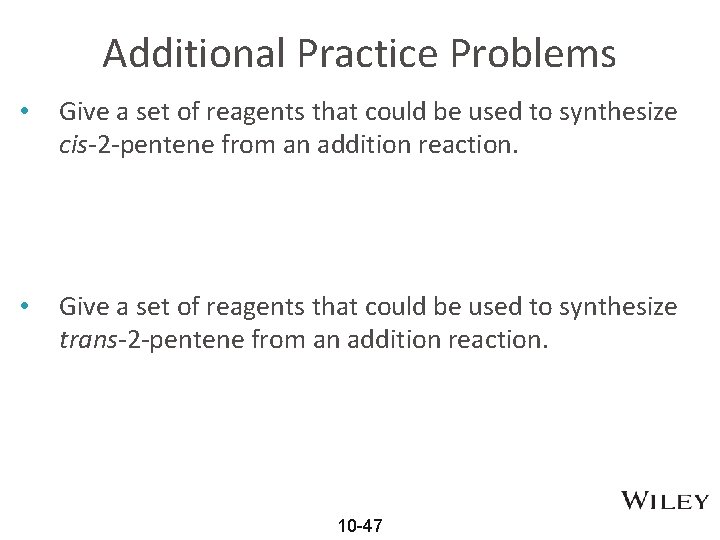
Additional Practice Problems • Give a set of reagents that could be used to synthesize cis-2 -pentene from an addition reaction. • Give a set of reagents that could be used to synthesize trans-2 -pentene from an addition reaction. 10 -47

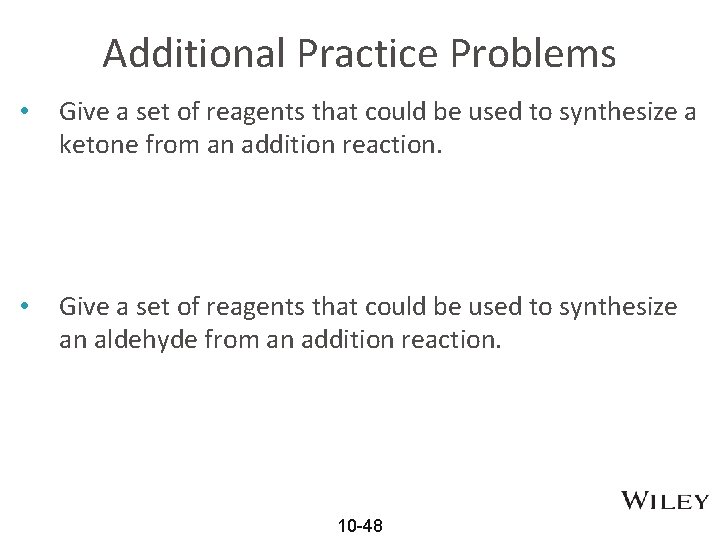
Additional Practice Problems • Give a set of reagents that could be used to synthesize a ketone from an addition reaction. • Give a set of reagents that could be used to synthesize an aldehyde from an addition reaction. 10 -48

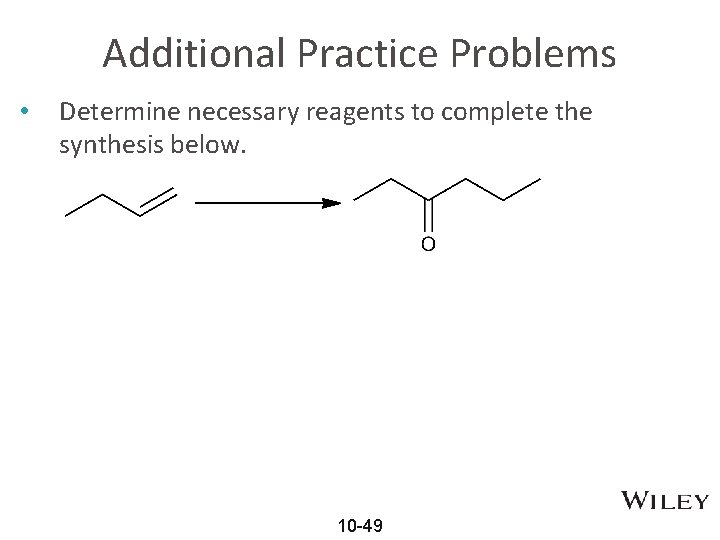
Additional Practice Problems • Determine necessary reagents to complete the synthesis below. 10 -49

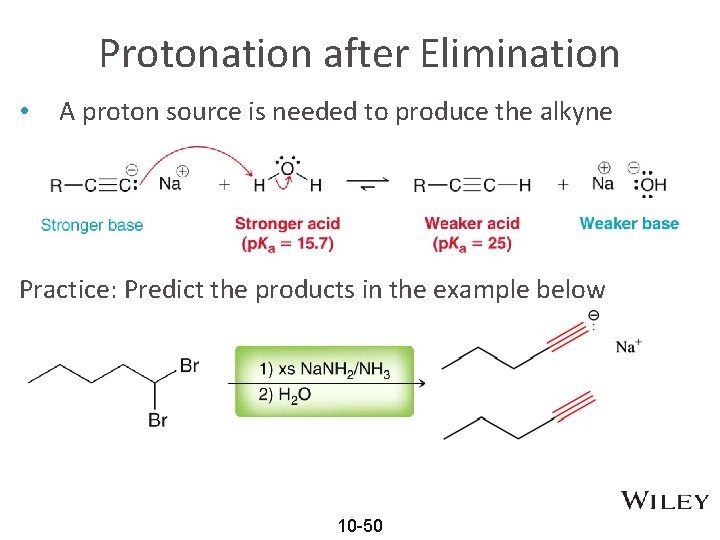
Protonation after Elimination • A proton source is needed to produce the alkyne Practice: Predict the products in the example below 10 -50

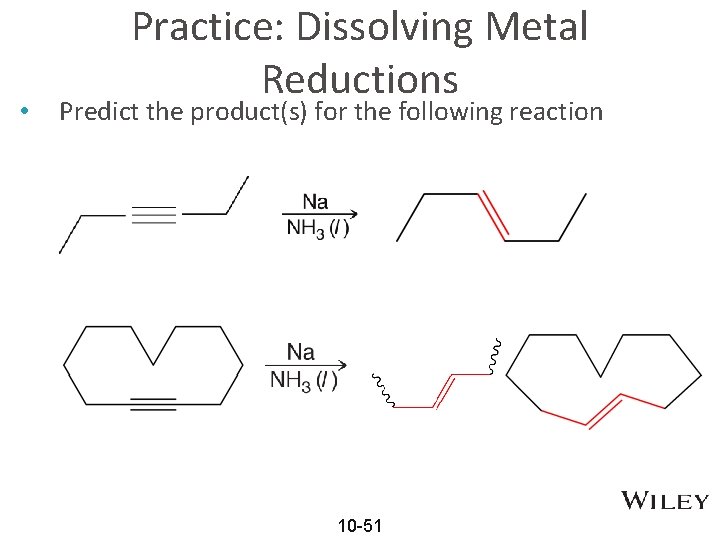
• Practice: Dissolving Metal Reductions Predict the product(s) for the following reaction 10 -51