Alkenes Principle reaction type electrophilic addition Mechanism carbocation

Alkenes Principle reaction type: electrophilic addition Mechanism: carbocation intermediate; cyclic intermediate
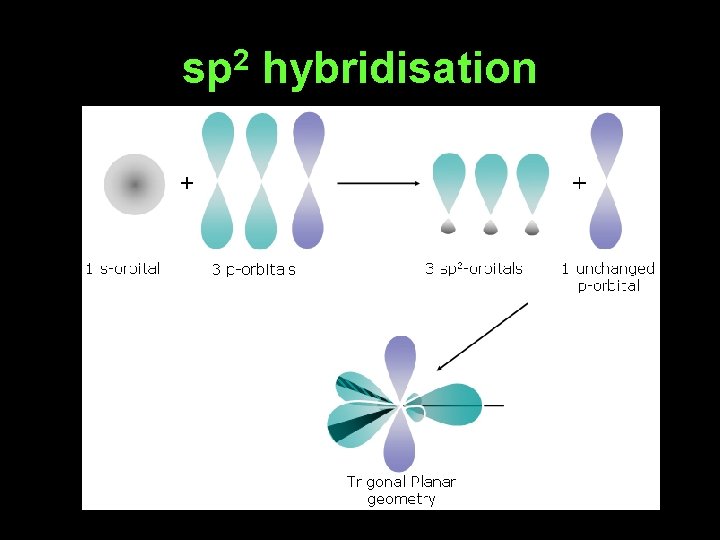
sp 2 hybridisation
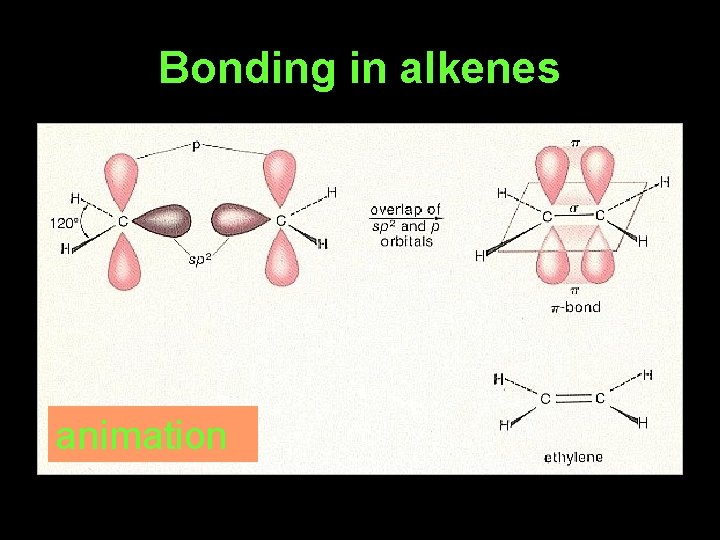
Bonding in alkenes animation

Synthesis of alkenes 1. From alcohols - acid-catalysed dehydration of alcohols 2. From halogenoalkanes - elimination reaction with potassium hydroxide in ethanolic solution
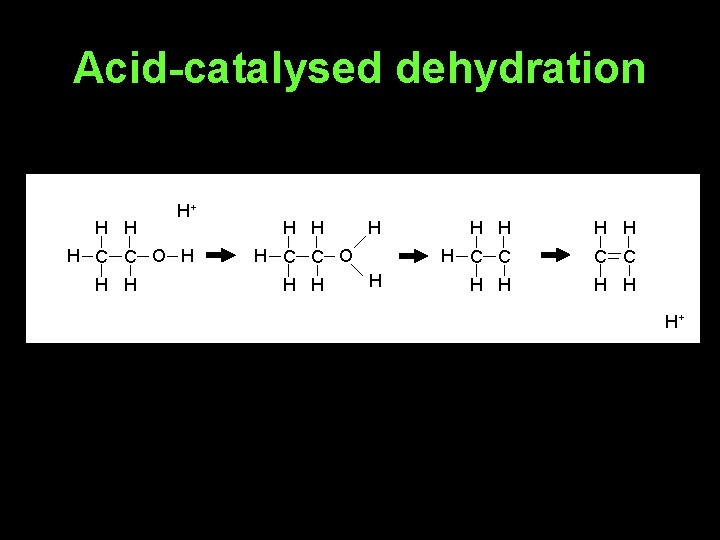
Acid-catalysed dehydration H H H+ H C C O H H H H C C H H H+
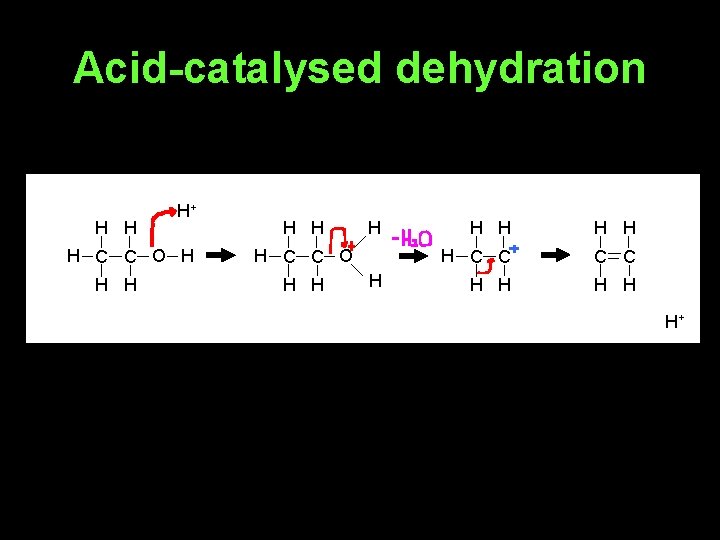
Acid-catalysed dehydration H H H+ H C C O H H H H C C H H H+
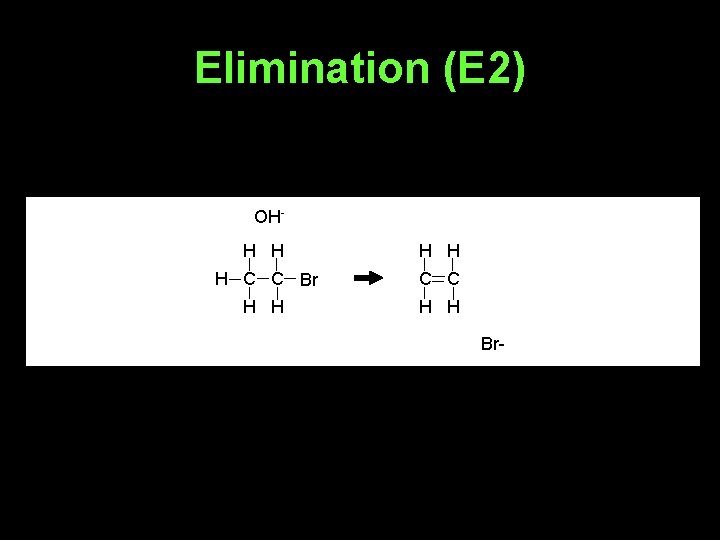
Elimination (E 2) OHH H H C C Br C C H H Br-
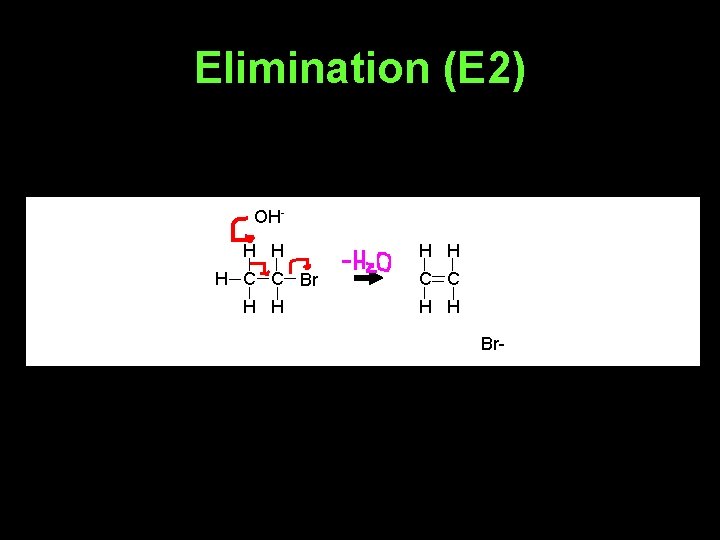
Elimination (E 2) OHH H H C C Br C C H H Br-
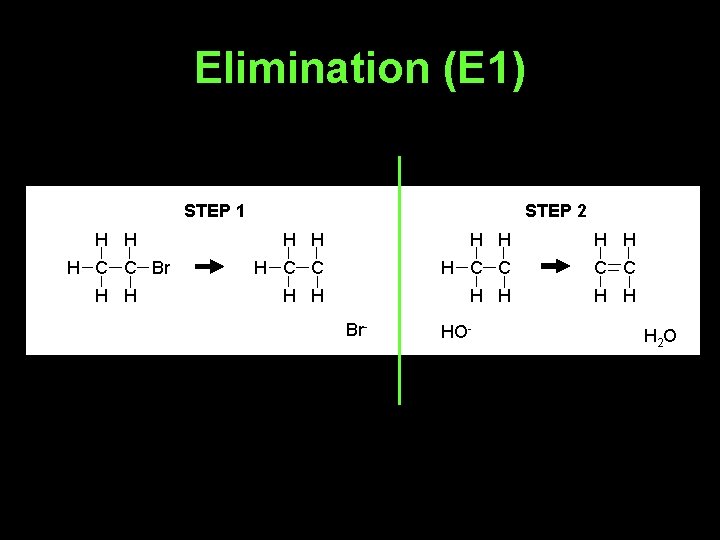
Elimination (E 1) STEP 1 H H H C C Br H H STEP 2 H H H H C C C C H H H Br- HO- H 2 O
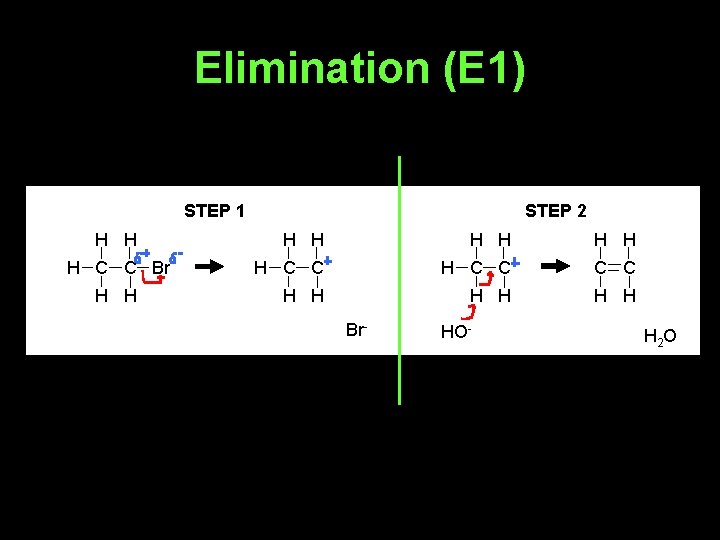
Elimination (E 1) STEP 1 H H H C C Br H H STEP 2 H H H H C C C C H H H Br- HO- H 2 O

• Whether E 2 or E 1 happens depends on the base used and the halogenoalkane involved.

Reactions of alkenes

Reactions of alkenes • Halogenation • Hydrohalogenation • Hydrogenation • (Acid-catalysed) Hydration
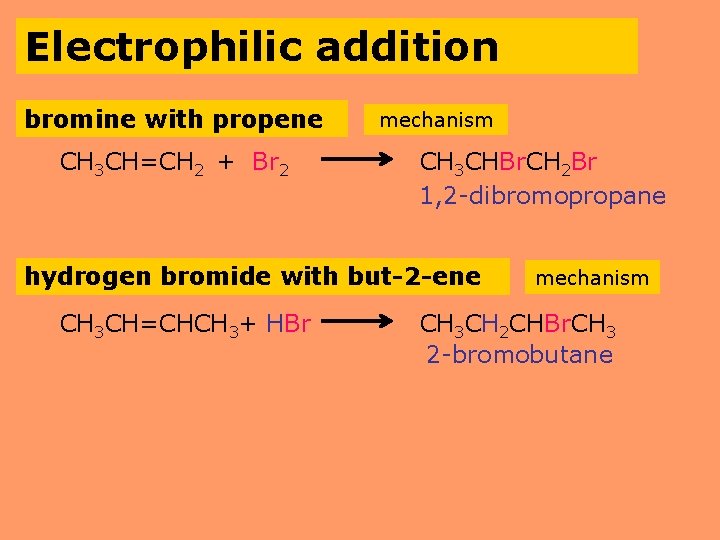
Electrophilic addition bromine with propene CH 3 CH=CH 2 + Br 2 mechanism CH 3 CHBr. CH 2 Br 1, 2 -dibromopropane hydrogen bromide with but-2 -ene CH 3 CH=CHCH 3+ HBr mechanism CH 3 CH 2 CHBr. CH 3 2 -bromobutane
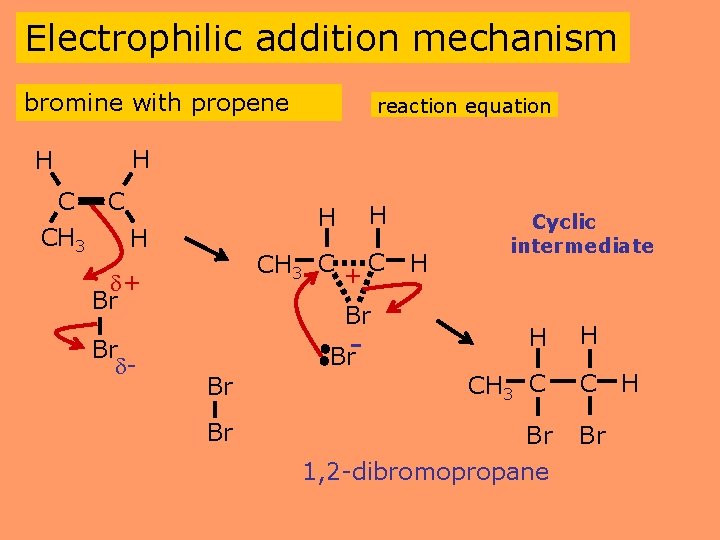
Electrophilic addition mechanism bromine with propene H reaction equation H C C CH 3 H CH 3 C + C + Br Br - H H Br H Cyclic intermediate H H Br CH 3 C C Br Br 1, 2 -dibromopropane Br- Br H
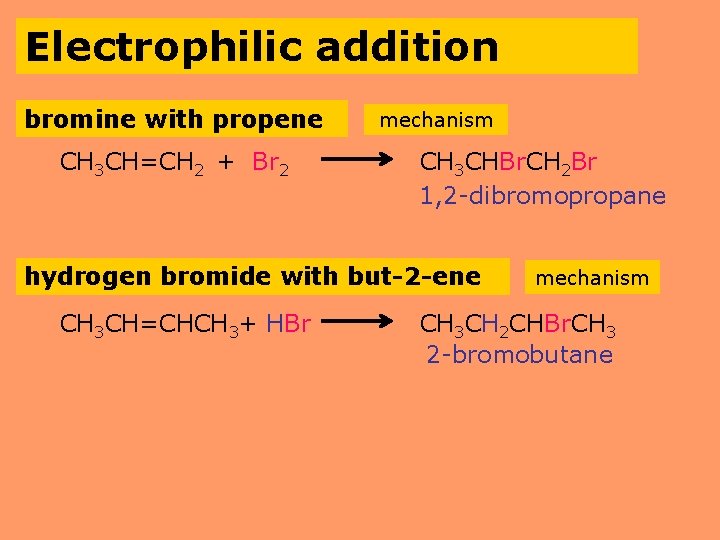
Electrophilic addition bromine with propene CH 3 CH=CH 2 + Br 2 mechanism CH 3 CHBr. CH 2 Br 1, 2 -dibromopropane hydrogen bromide with but-2 -ene CH 3 CH=CHCH 3+ HBr mechanism CH 3 CH 2 CHBr. CH 3 2 -bromobutane
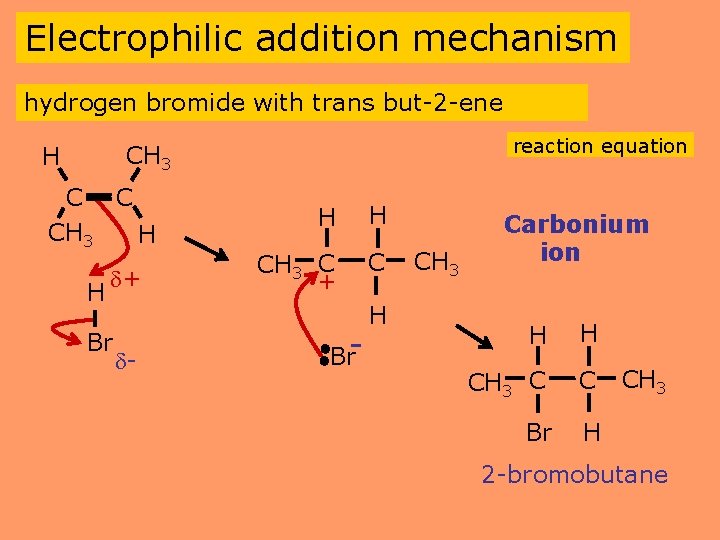
Electrophilic addition mechanism hydrogen bromide with trans but-2 -ene reaction equation CH 3 H C C CH 3 H H + Br - H H CH 3 C + C Br- H CH 3 Carbonium ion H H CH 3 C C Br H CH 3 2 -bromobutane

Markovnikov’s Rule When a compound HX is added across a C=C, the hydrogen atom bonds to the carbon with the most hydrogen atoms already bonded.
- Slides: 18