Alkenes Bonding The electron configuration of carbon is

Alkenes
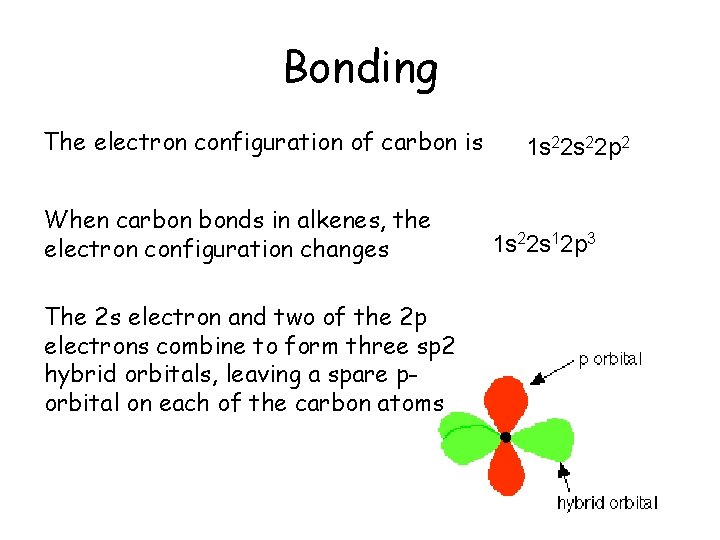
Bonding The electron configuration of carbon is When carbon bonds in alkenes, the electron configuration changes The 2 s electron and two of the 2 p electrons combine to form three sp 2 hybrid orbitals, leaving a spare porbital on each of the carbon atoms 1 s 22 p 2 1 s 22 s 12 p 3
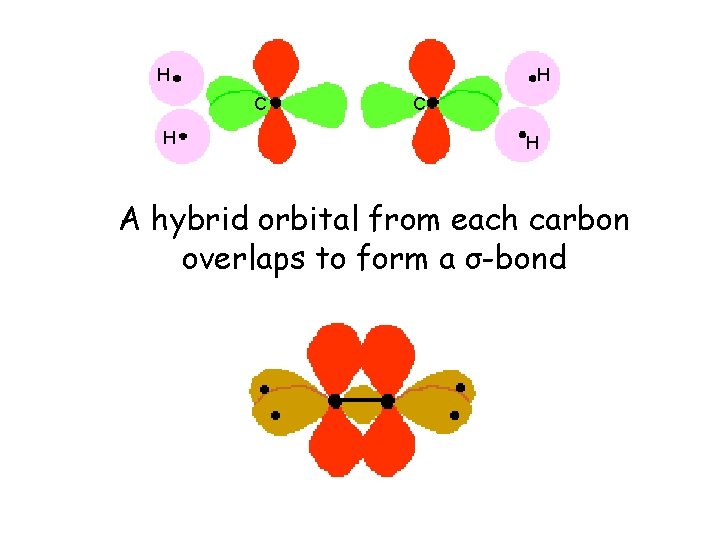
H H C H A hybrid orbital from each carbon overlaps to form a σ-bond
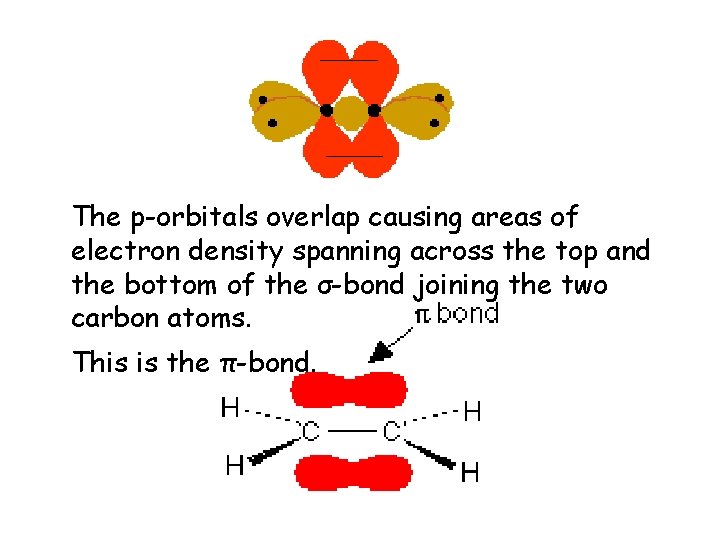
The p-orbitals overlap causing areas of electron density spanning across the top and the bottom of the σ-bond joining the two carbon atoms. This is the π-bond.

Test for unsaturation • If an alkene is shaken with bromine water (Br 2) the colour changes from reddy-brown to colourless as dihalogenoalkanes are formed.
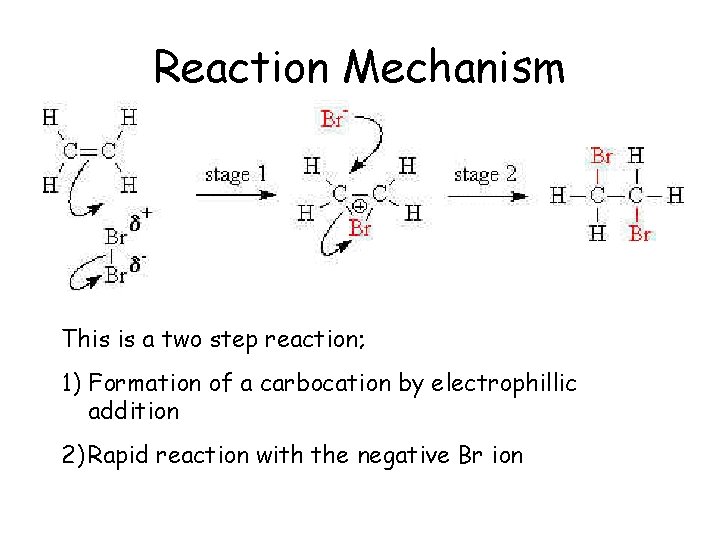
Reaction Mechanism This is a two step reaction; 1) Formation of a carbocation by electrophillic addition 2) Rapid reaction with the negative Br ion

Reactions with hydrogen halides If an alkene is bubbled through concentrated HCl(aq) at room temperature, a monosubstituted chloro-alkane is formed.

Addition of steam When steam and a gaseous alkene are passed over a solid phosphoric acid catalyst (H 3 PO 4) at 600 K and 6 MPa, alcohols are formed.

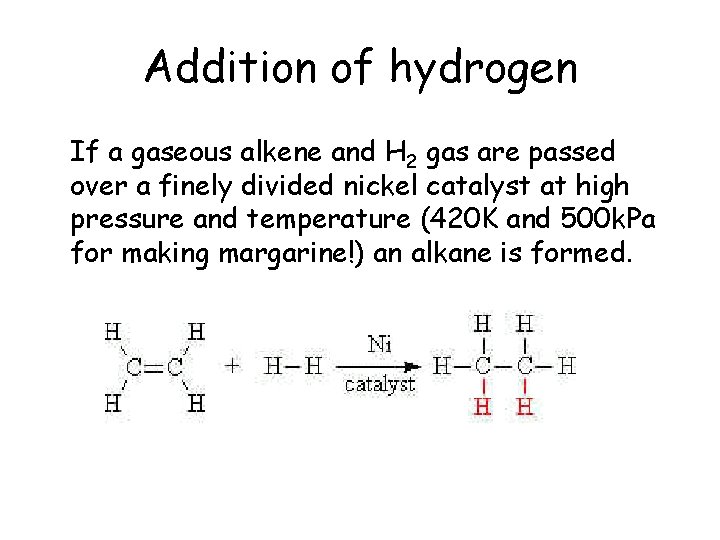
Addition of hydrogen If a gaseous alkene and H 2 gas are passed over a finely divided nickel catalyst at high pressure and temperature (420 K and 500 k. Pa for making margarine!) an alkane is formed.
- Slides: 10