Alkene st 1 stage Wrea Mohammed Alkene Alkenes
![ØStep [1] Find the longest chain that contains both carbon atoms of the double ØStep [1] Find the longest chain that contains both carbon atoms of the double](https://slidetodoc.com/presentation_image_h2/05587722f692707a16d233552399a118/image-11.jpg)
![Ø Step [2] Number the carbon chain to give the double bond the lower Ø Step [2] Number the carbon chain to give the double bond the lower](https://slidetodoc.com/presentation_image_h2/05587722f692707a16d233552399a118/image-13.jpg)

![Ø Step [3] Write a single word name for the alkene Ø Combine the Ø Step [3] Write a single word name for the alkene Ø Combine the](https://slidetodoc.com/presentation_image_h2/05587722f692707a16d233552399a118/image-15.jpg)

- Slides: 28

Alkene st 1 stage Wrea Mohammed

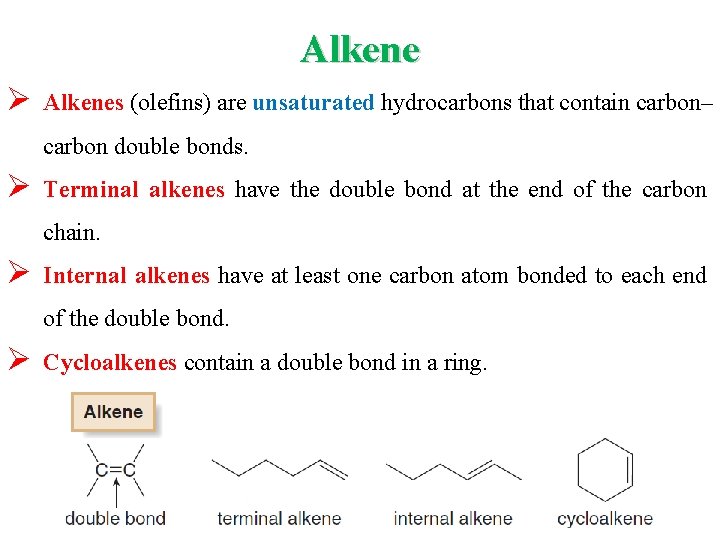
Alkene Ø Alkenes (olefins) are unsaturated hydrocarbons that contain carbon– carbon double bonds. Ø Terminal alkenes have the double bond at the end of the carbon chain. Ø Internal alkenes have at least one carbon atom bonded to each end of the double bond. Ø Cycloalkenes contain a double bond in a ring.

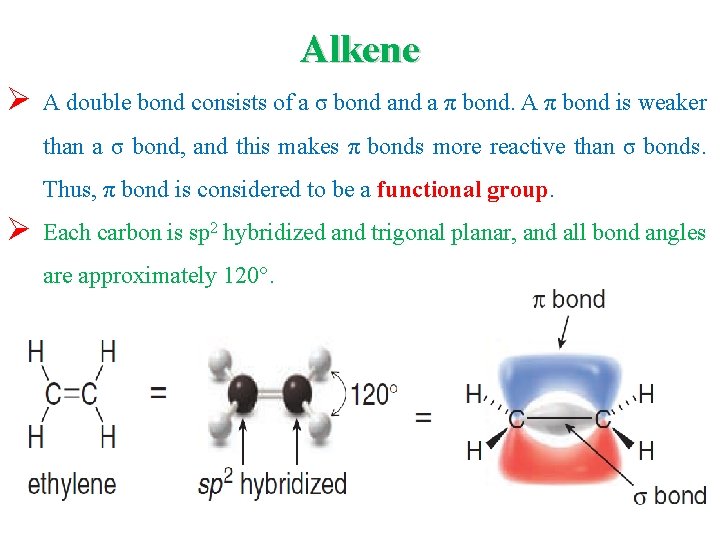
Alkene Ø A double bond consists of a σ bond a π bond. A π bond is weaker than a σ bond, and this makes π bonds more reactive than σ bonds. Thus, π bond is considered to be a functional group. Ø Each carbon is sp 2 hybridized and trigonal planar, and all bond angles are approximately 120°.

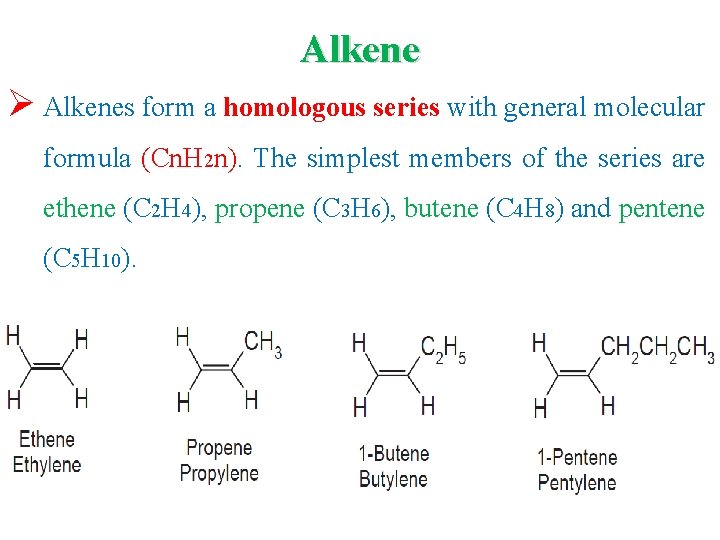
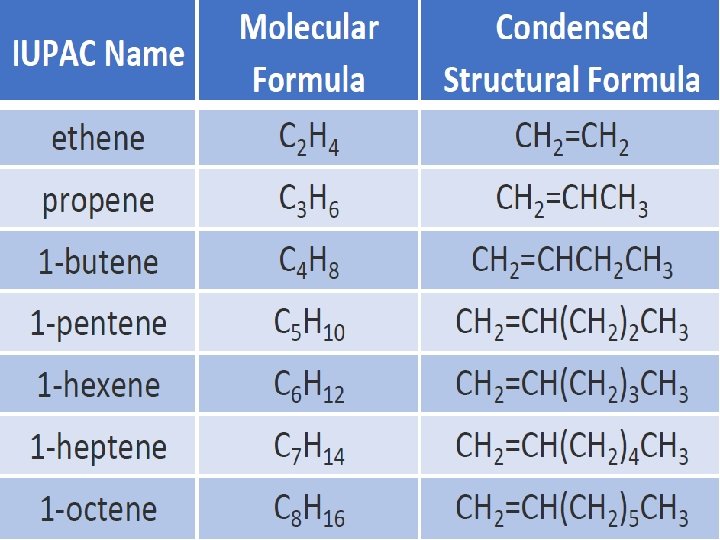
Alkene Ø Alkenes form a homologous series with general molecular formula (Cn. H 2 n). The simplest members of the series are ethene (C 2 H 4), propene (C 3 H 6), butene (C 4 H 8) and pentene (C 5 H 10).


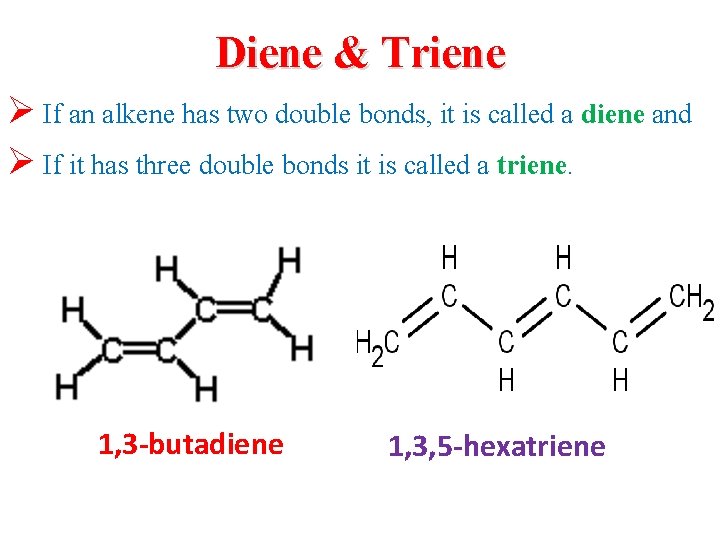
Diene & Triene Ø If an alkene has two double bonds, it is called a diene and Ø If it has three double bonds it is called a triene. 1, 3 -butadiene 1, 3, 5 -hexatriene

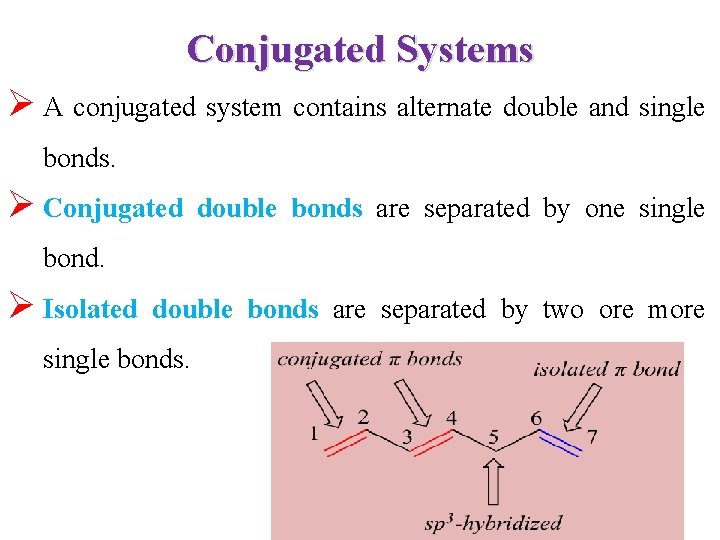
Conjugated Systems Ø A conjugated system contains alternate double and single bonds. Ø Conjugated double bonds are separated by one single bond. Ø Isolated double bonds are separated by two ore more single bonds.

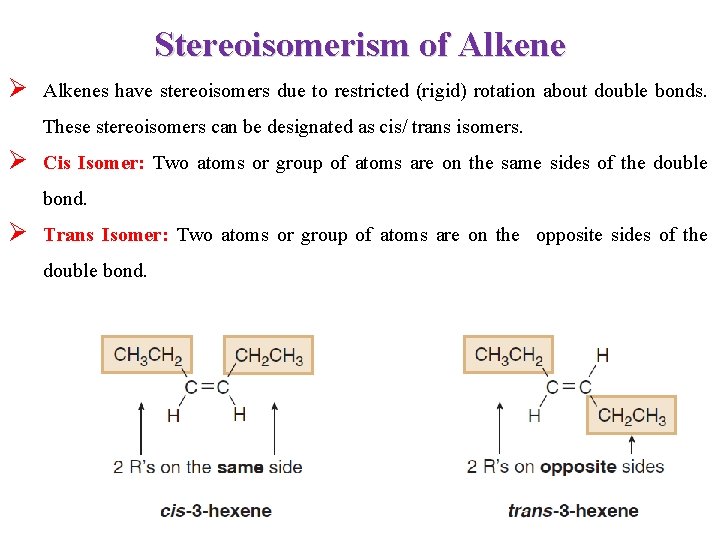
Stereoisomerism of Alkene Ø Alkenes have stereoisomers due to restricted (rigid) rotation about double bonds. These stereoisomers can be designated as cis/ trans isomers. Ø Cis Isomer: Two atoms or group of atoms are on the same sides of the double bond. Ø Trans Isomer: Two atoms or group of atoms are on the opposite sides of the double bond.

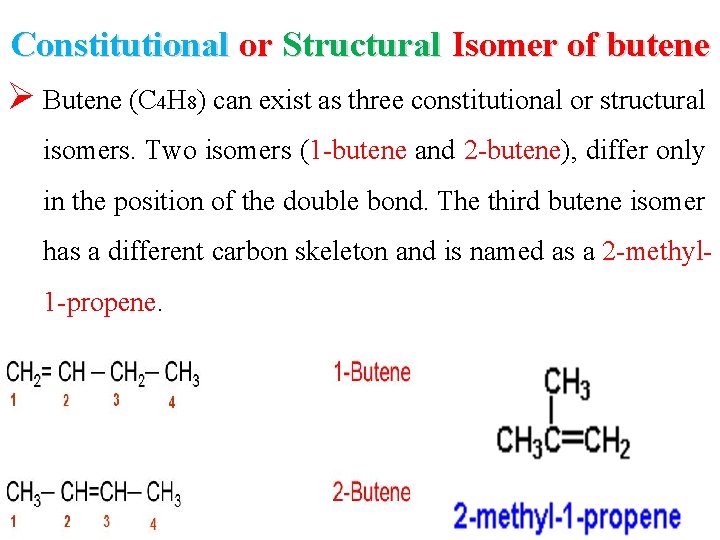
Constitutional or Structural Isomer of butene Ø Butene (C 4 H 8) can exist as three constitutional or structural isomers. Two isomers (1 -butene and 2 -butene), differ only in the position of the double bond. The third butene isomer has a different carbon skeleton and is named as a 2 -methyl 1 -propene.

Nomenclature of Alkene ØIn the IUPAC system, an alkene is identified by the suffix -ene.
![ØStep 1 Find the longest chain that contains both carbon atoms of the double ØStep [1] Find the longest chain that contains both carbon atoms of the double](https://slidetodoc.com/presentation_image_h2/05587722f692707a16d233552399a118/image-11.jpg)
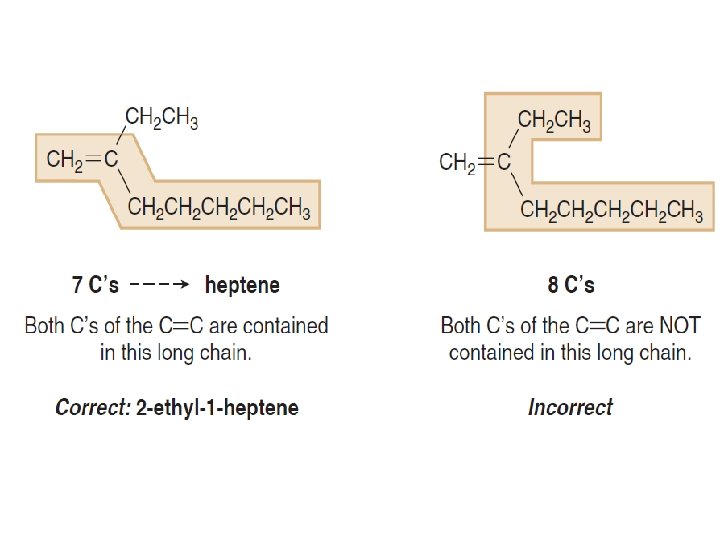
ØStep [1] Find the longest chain that contains both carbon atoms of the double bond.

![Ø Step 2 Number the carbon chain to give the double bond the lower Ø Step [2] Number the carbon chain to give the double bond the lower](https://slidetodoc.com/presentation_image_h2/05587722f692707a16d233552399a118/image-13.jpg)
Ø Step [2] Number the carbon chain to give the double bond the lower number. Ø The atoms are numbered starting from the end nearest to the double bond.

ØIf the double bond is the same distance from both ends, begin with the end nearer the first branch point.
![Ø Step 3 Write a single word name for the alkene Ø Combine the Ø Step [3] Write a single word name for the alkene Ø Combine the](https://slidetodoc.com/presentation_image_h2/05587722f692707a16d233552399a118/image-15.jpg)
Ø Step [3] Write a single word name for the alkene Ø Combine the numbers and names of all substituents with the parent name to form one word. Use hyphens (-) to separate numbers from names. Use commas (, ) to separate numbers. Ø If two or more substituents are attached to the parent chain, write them in alphabetical order. If two or more substituents are identical, use prefixes (di-, tri-, tetra-, penta-, etc. ). Each substituent must have a number, even if the numbers must be repeated. Ø Indicate the position of the double bond by giving the number of the first carbon of the double bond before the -ene ending. If more than one double bond is present use the suffixes -diene, triene, etc.

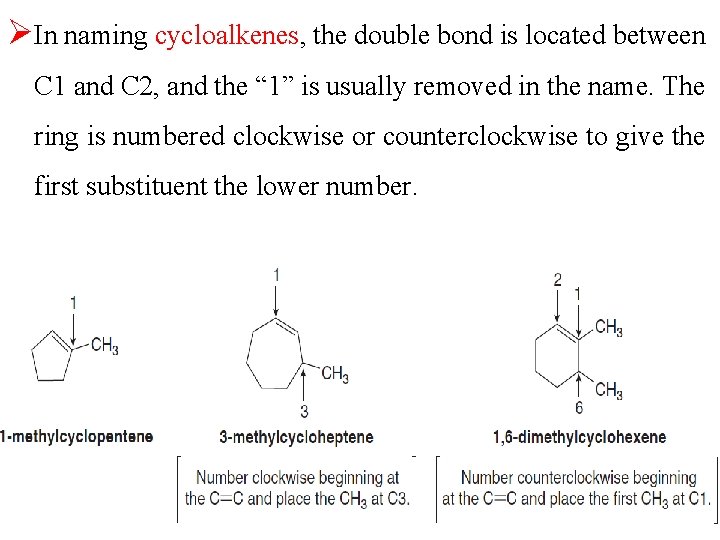
ØIn naming cycloalkenes, the double bond is located between C 1 and C 2, and the “ 1” is usually removed in the name. The ring is numbered clockwise or counterclockwise to give the first substituent the lower number.

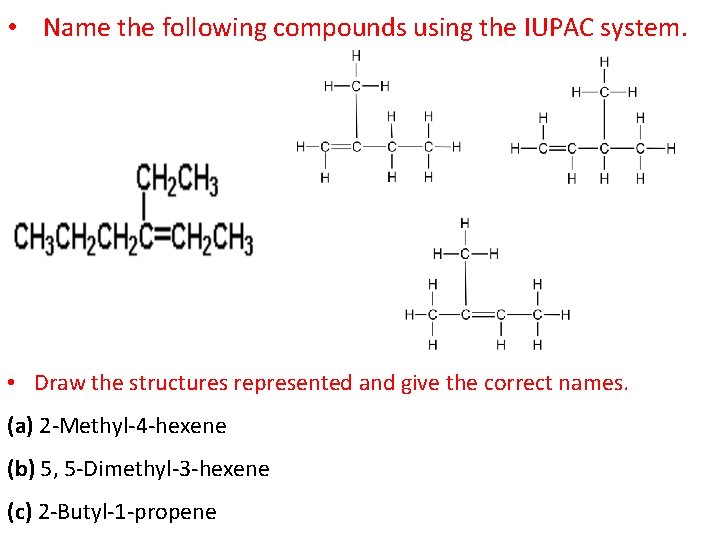
• Name the following compounds using the IUPAC system. • Draw the structures represented and give the correct names. (a) 2 -Methyl-4 -hexene (b) 5, 5 -Dimethyl-3 -hexene (c) 2 -Butyl-1 -propene

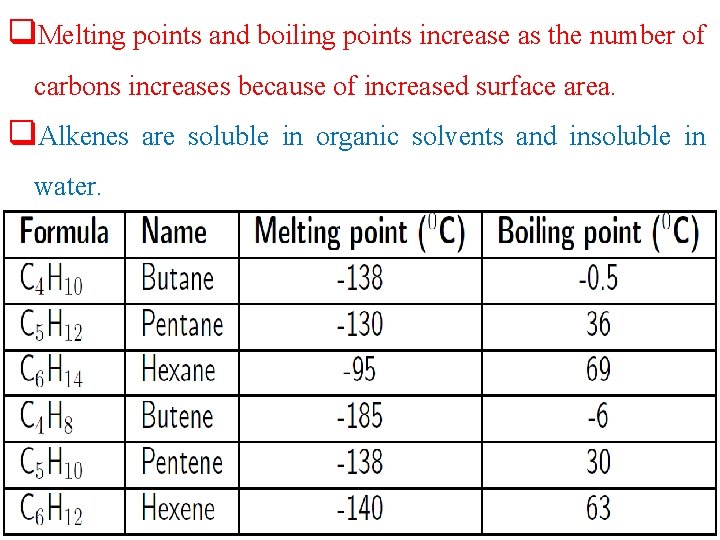
Physical properties of alkenes q. The properties of the alkenes are very similar to alkanes, except that the alkenes are more reactive because they are unsaturated. q Most alkenes exhibit only weak van der Waals interactions. q. Alkenes have low melting points and boiling points.

q. Melting points and boiling points increase as the number of carbons increases because of increased surface area. q. Alkenes are soluble in organic solvents and insoluble in water.

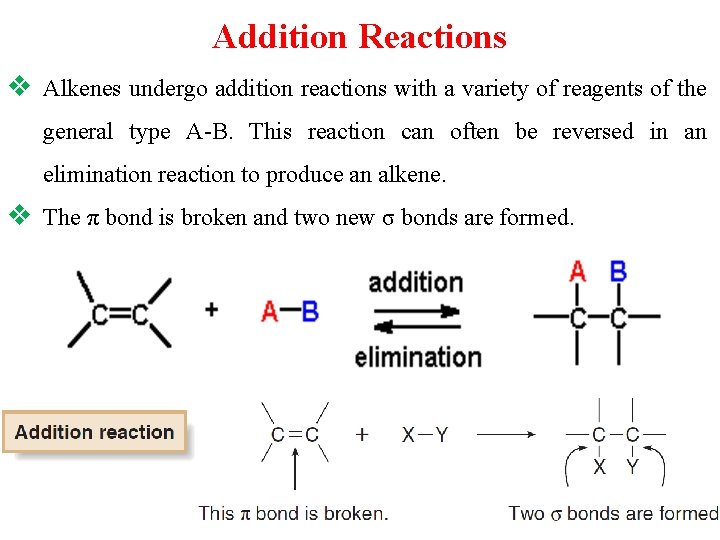
Reactions of Alkenes: v. The important reactions of alkenes reveals the two basic types of reactions of alkenes - addition and oxidation. v. Because the C – C π bond of an alkene is much weaker than a C – C σ bond, the characteristic reaction of alkenes is addition: the π bond is broken and two new σ bonds are formed.

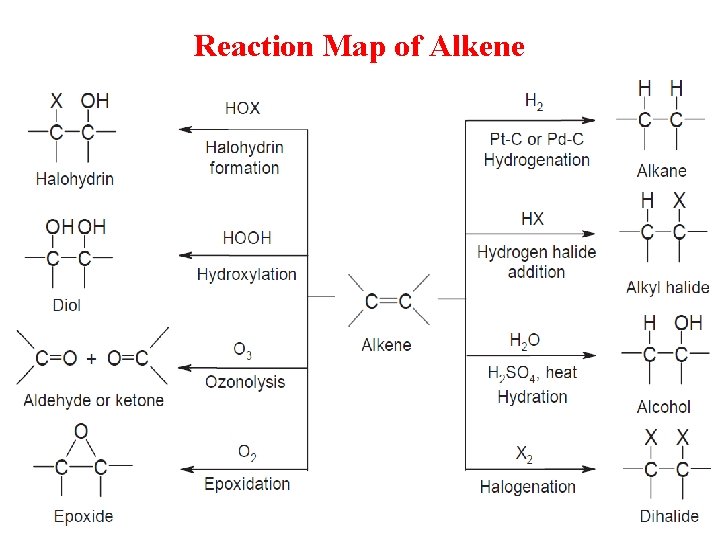
Reaction Map of Alkene

Addition Reactions v Alkenes undergo addition reactions with a variety of reagents of the general type A-B. This reaction can often be reversed in an elimination reaction to produce an alkene. v The π bond is broken and two new σ bonds are formed.

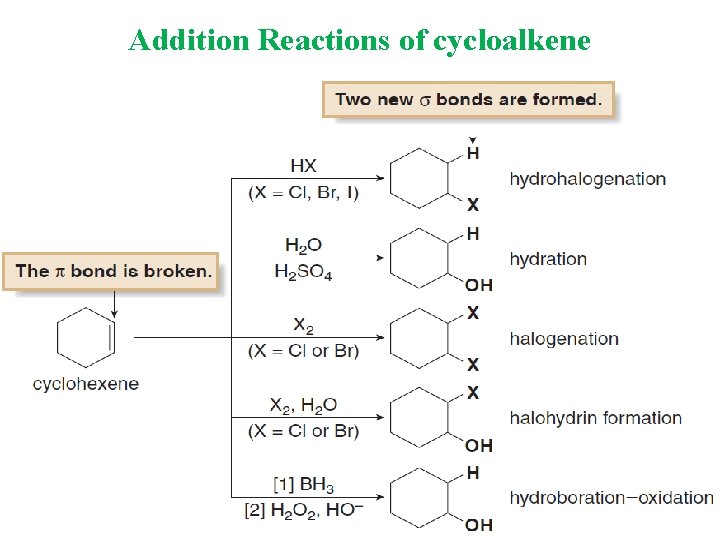
Addition Reactions of cycloalkene

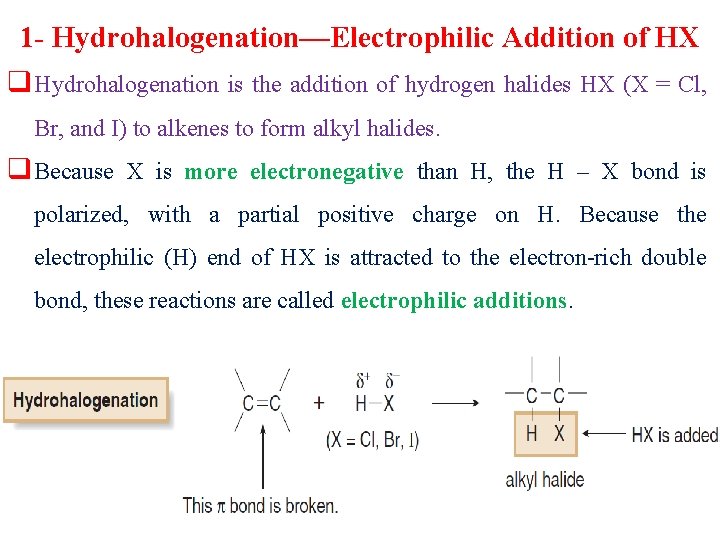
1 - Hydrohalogenation—Electrophilic Addition of HX q Hydrohalogenation is the addition of hydrogen halides HX (X = Cl, Br, and I) to alkenes to form alkyl halides. q Because X is more electronegative than H, the H – X bond is polarized, with a partial positive charge on H. Because the electrophilic (H) end of HX is attracted to the electron-rich double bond, these reactions are called electrophilic additions.

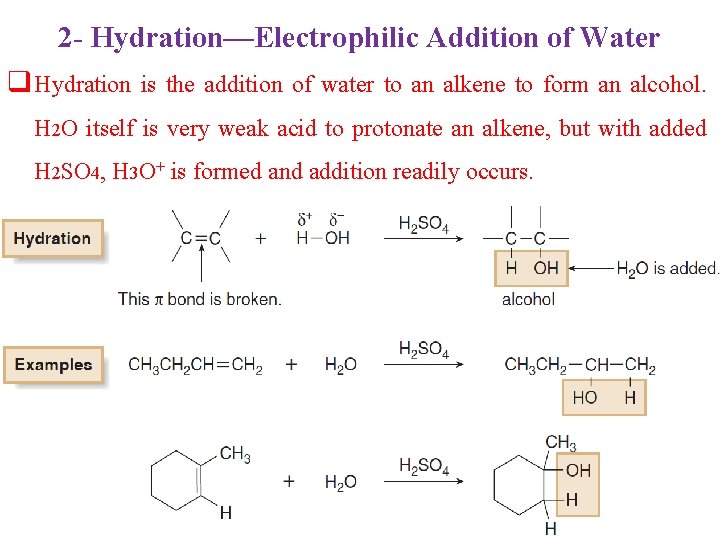
2 - Hydration—Electrophilic Addition of Water q Hydration is the addition of water to an alkene to form an alcohol. H 2 O itself is very weak acid to protonate an alkene, but with added H 2 SO 4, H 3 O+ is formed and addition readily occurs.

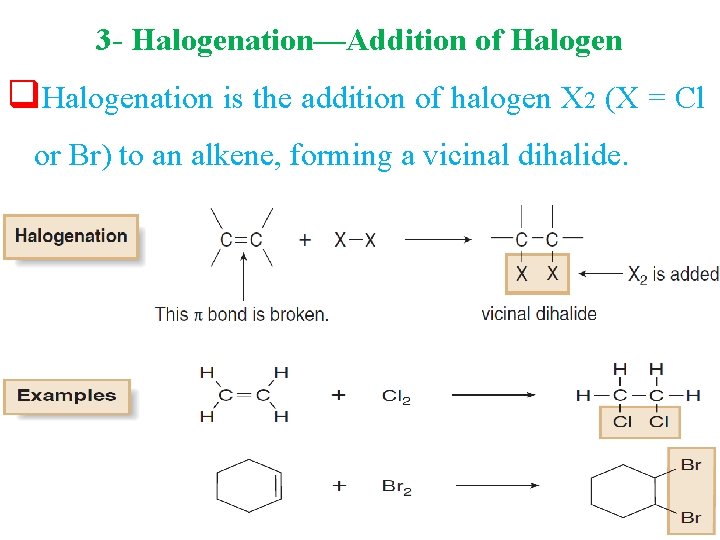
3 - Halogenation—Addition of Halogen q. Halogenation is the addition of halogen X 2 (X = Cl or Br) to an alkene, forming a vicinal dihalide.

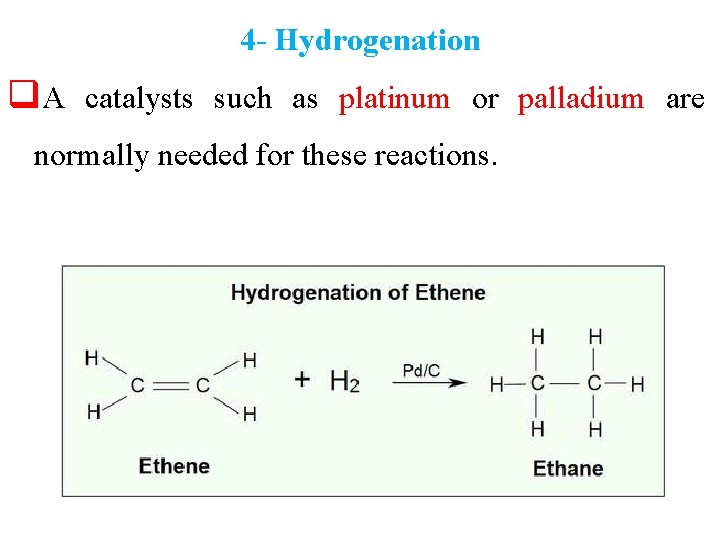
4 - Hydrogenation q. A catalysts such as platinum or palladium are normally needed for these reactions.

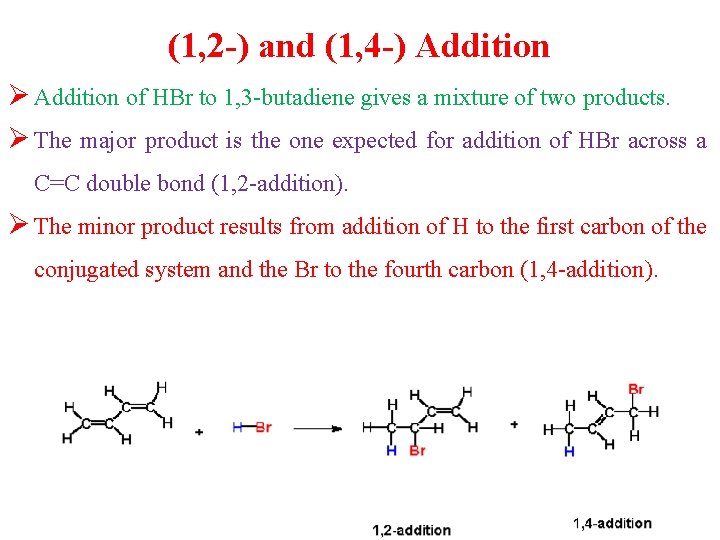
(1, 2 -) and (1, 4 -) Addition Ø Addition of HBr to 1, 3 -butadiene gives a mixture of two products. Ø The major product is the one expected for addition of HBr across a C=C double bond (1, 2 -addition). Ø The minor product results from addition of H to the first carbon of the conjugated system and the Br to the fourth carbon (1, 4 -addition).