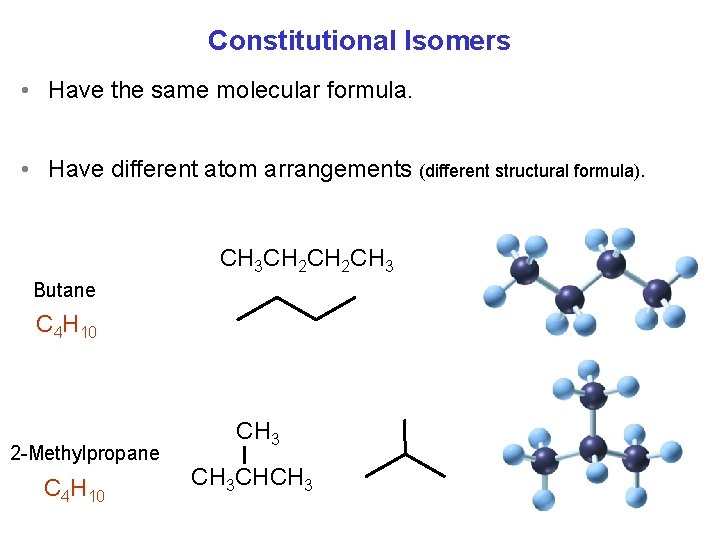
Alkanes Constitutional Isomers Have the same molecular formula









- Slides: 24

Alkanes

Constitutional Isomers • Have the same molecular formula. • Have different atom arrangements (different structural formula). CH 3 CH 2 CH 3 Butane C 4 H 10 2 -Methylpropane C 4 H 10 CH 3 CHCH 3

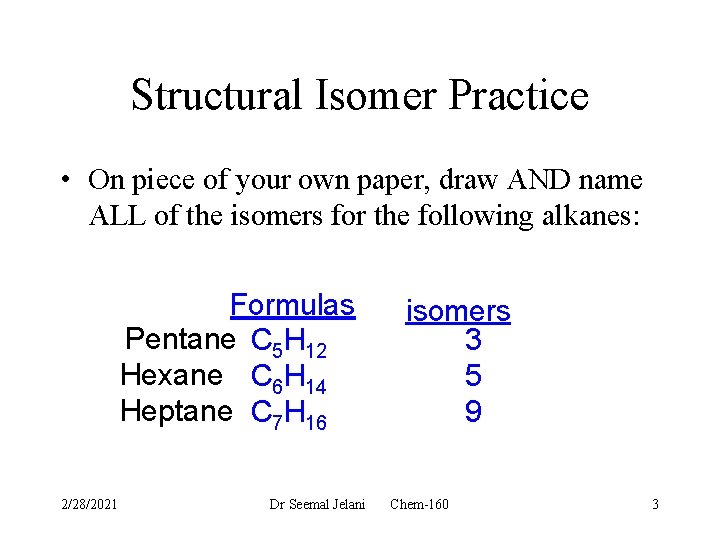
Structural Isomer Practice • On piece of your own paper, draw AND name ALL of the isomers for the following alkanes: Formulas Pentane C 5 H 12 Hexane C 6 H 14 Heptane C 7 H 16 2/28/2021 Dr Seemal Jelani isomers 3 5 9 Chem-160 3

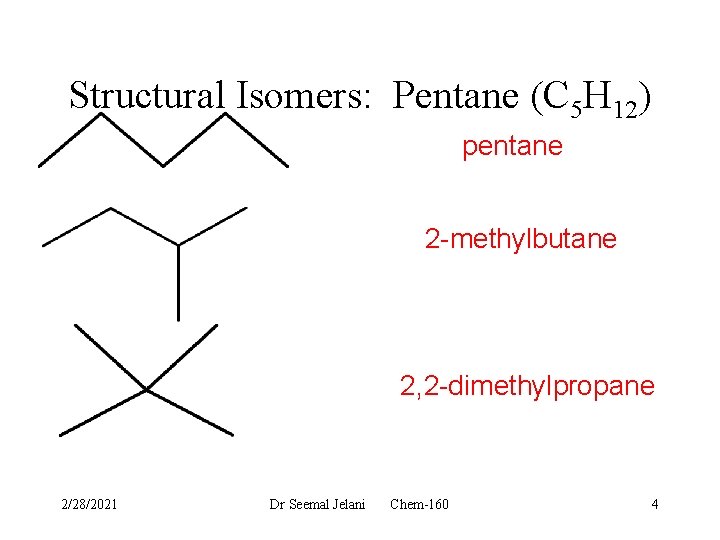
Structural Isomers: Pentane (C 5 H 12) pentane 2 -methylbutane 2, 2 -dimethylpropane 2/28/2021 Dr Seemal Jelani Chem-160 4

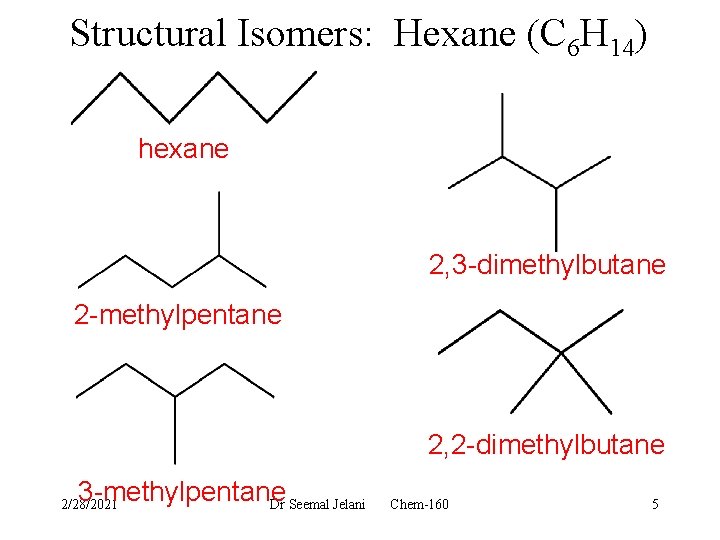
Structural Isomers: Hexane (C 6 H 14) hexane 2, 3 -dimethylbutane 2 -methylpentane 2, 2 -dimethylbutane 3 -methylpentane Dr Seemal Jelani 2/28/2021 Chem-160 5

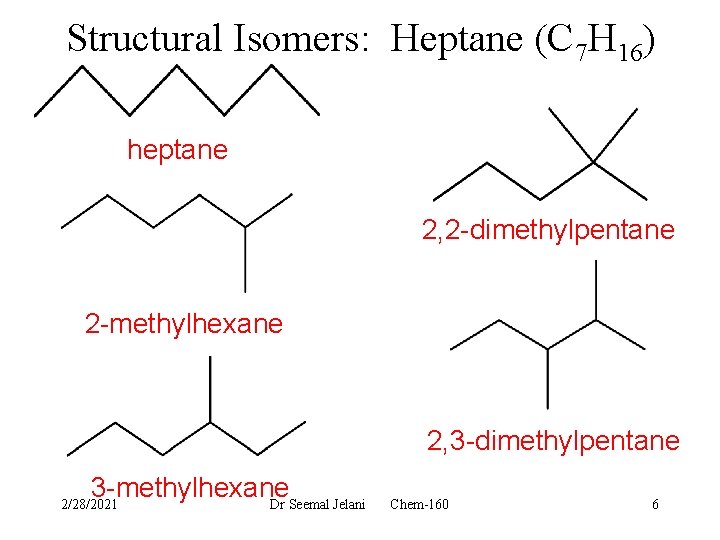
Structural Isomers: Heptane (C 7 H 16) heptane 2, 2 -dimethylpentane 2 -methylhexane 2, 3 -dimethylpentane 3 -methylhexane Dr Seemal Jelani 2/28/2021 Chem-160 6

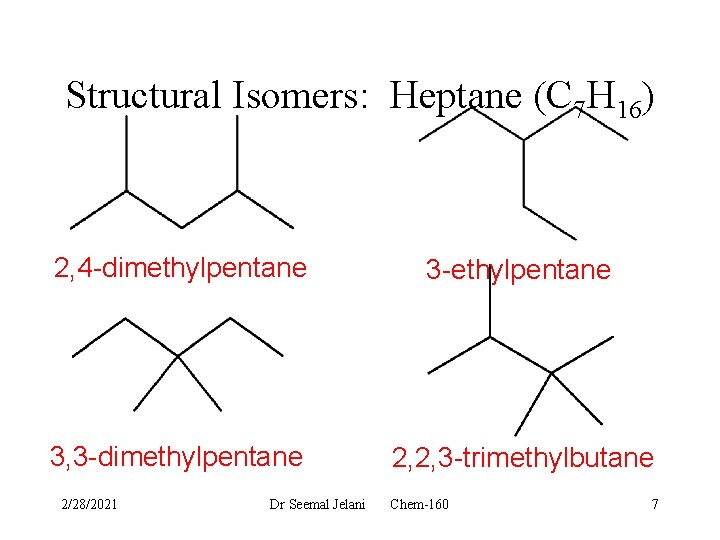
Structural Isomers: Heptane (C 7 H 16) 2, 4 -dimethylpentane 3 -ethylpentane 3, 3 -dimethylpentane 2, 2, 3 -trimethylbutane 2/28/2021 Dr Seemal Jelani Chem-160 7

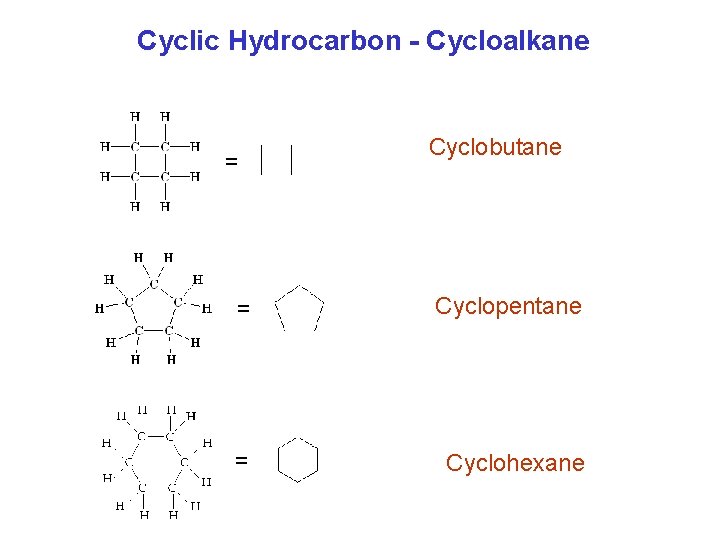
Cyclic Hydrocarbon - Cycloalkane = Cyclobutane = Cyclopentane = Cyclohexane

Physical Properties of Alkanes • • • Nonpolar Insoluble in water. Lower density than water. Low boiling and melting points. Gases with 1 -4 carbon atoms. (methane, propane, butane) • Liquids with 5 -17 carbon atoms. (kerosene, diesel, and jet fuels) • Solids with 18 or more carbon atoms. (wax, paraffin, Vaseline)

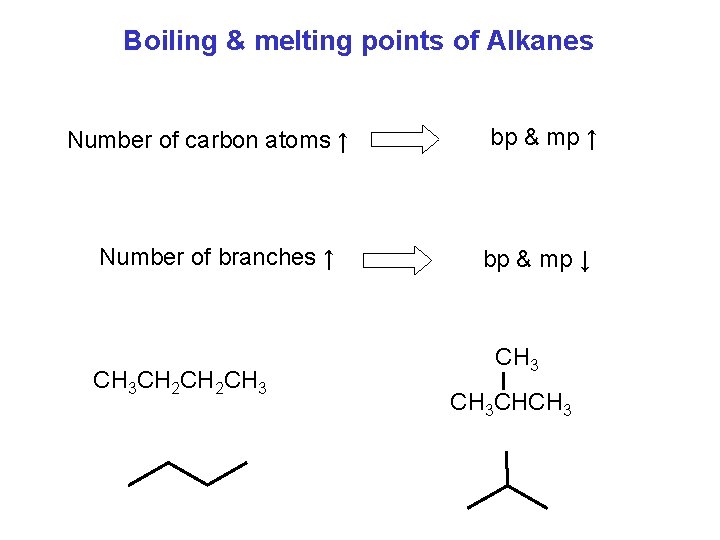
Boiling & melting points of Alkanes Number of carbon atoms ↑ Number of branches ↑ CH 3 CH 2 CH 3 bp & mp ↑ bp & mp ↓ CH 3 CHCH 3

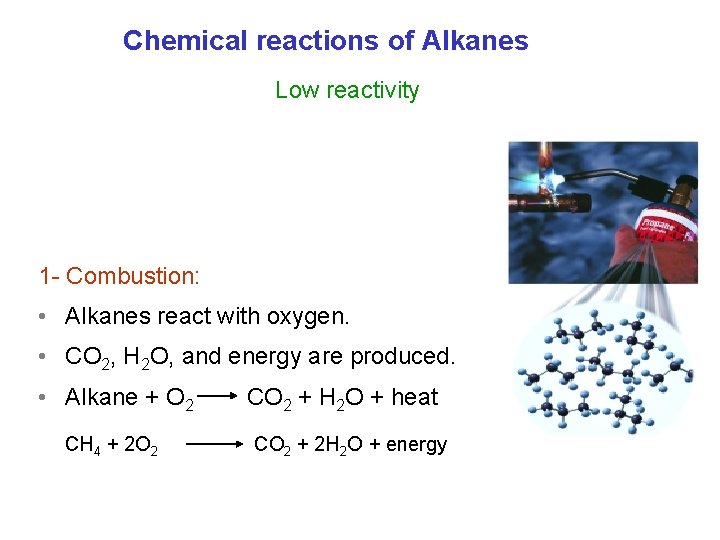
Chemical reactions of Alkanes Low reactivity 1 - Combustion: • Alkanes react with oxygen. • CO 2, H 2 O, and energy are produced. • Alkane + O 2 CH 4 + 2 O 2 CO 2 + H 2 O + heat CO 2 + 2 H 2 O + energy

Halogenation Free Radical reaction 2/28/2021 Dr Seemal Jelani Chem-160 12

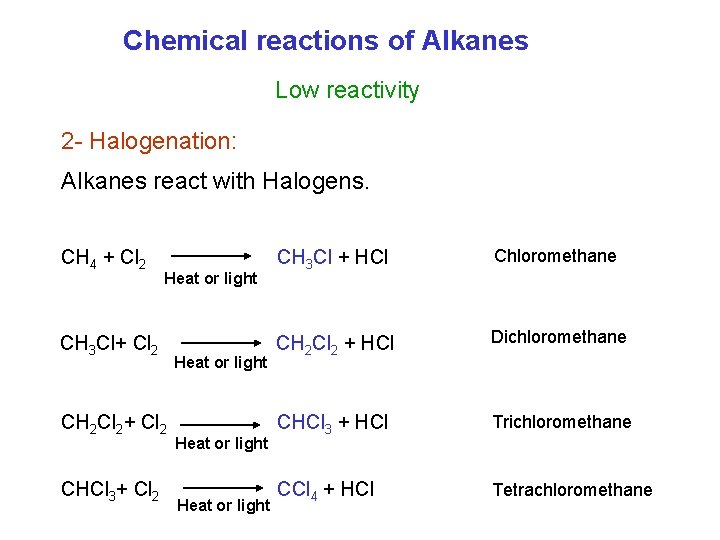
Chemical reactions of Alkanes Low reactivity 2 - Halogenation: Alkanes react with Halogens. CH 4 + Cl 2 Heat or light CH 3 Cl+ Cl 2 CH 2 Cl 2+ Cl 2 CHCl 3+ Cl 2 Heat or light CH 3 Cl + HCl Chloromethane CH 2 Cl 2 + HCl Dichloromethane CHCl 3 + HCl Trichloromethane CCl 4 + HCl Tetrachloromethane

Halogenation • Reaction between a substance and a halogen in which one or more halogens atoms are incorporated into molecules of the substance • Product is Hydrocarbon derivative • Halogenation is an example of Substitution reaction It is a reaction in which part of a small reacting molecule replaces an atom or group of atoms on a hydrocarbons 2/28/2021 Dr Seemal Jelani Chem-160 14

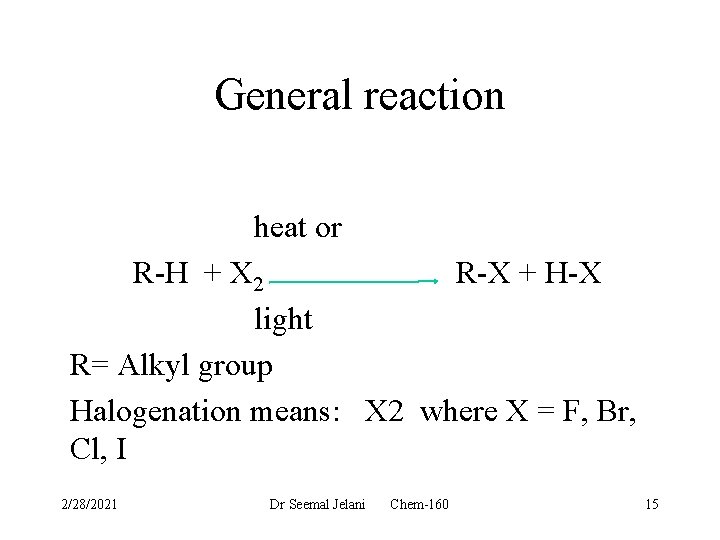
General reaction heat or R-H + X 2 R-X + H-X light R= Alkyl group Halogenation means: X 2 where X = F, Br, Cl, I 2/28/2021 Dr Seemal Jelani Chem-160 15

Radical Halogenation Terms • Mechanism – How the reaction occurs through multiple steps (most reactions actually occur in many steps) • Chain Reaction – Reactions that occur on their own after some initiating event • Free Radicals – Atoms that have one free electron—highly reactive 2/28/2021 Dr Seemal Jelani Chem-160 16

Radical Halogenation Terms • Initiation Step – Step where a bond is split by heat/light, producing free radicals • Propagation Step – Step where free radicals react with non-radicals, producing more free radicals and continuing the “chain reaction” • Termination Step – Step where free radicals react with each other, producing nonradicals and terminating the “chain reaction” 2/28/2021 Dr Seemal Jelani Chem-160 17

Initiation • Reaction starts with one photon of light. • • • Must be UV Wavelength less than 400 nm Energy less than 300 k. J per mole Reaction proceeds to completion after this. Reason is the chlorine molecule Light breaks the Cl-Cl bond • Homolytic fission Each atom takes an electron Forms radicals. 2/28/2021 Dr Seemal Jelani Chem-160 18

Initiation • • Homolytic fission forms radicals Radicals are very reactive atoms or groups. Cl 2 2 Cl. Radicals react quickly with surrounding molecules. 2/28/2021 Dr Seemal Jelani Chem-160 19

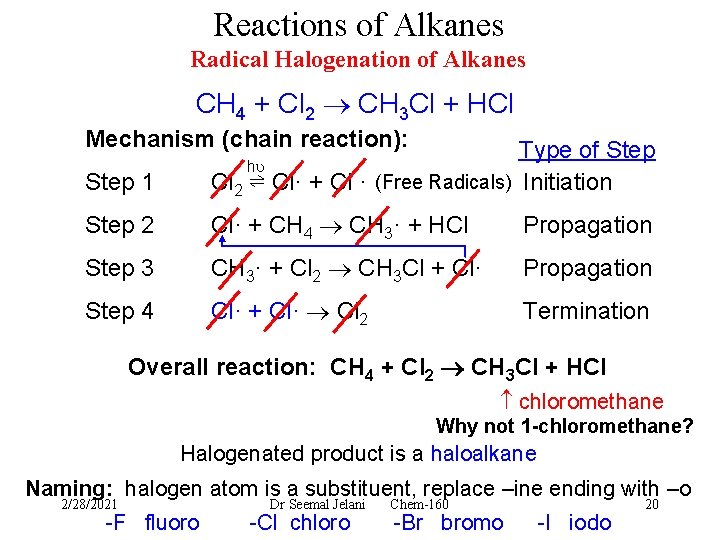
Reactions of Alkanes Radical Halogenation of Alkanes CH 4 + Cl 2 CH 3 Cl + HCl Mechanism (chain reaction): Step 1 Type of Step Cl 2 ⇌ Cl· + Cl · (Free Radicals) Initiation Step 2 Cl· + CH 4 CH 3· + HCl Propagation Step 3 CH 3· + Cl 2 CH 3 Cl + Cl· Propagation Step 4 Cl· + Cl· Cl 2 Termination h Overall reaction: CH 4 + Cl 2 CH 3 Cl + HCl chloromethane Why not 1 -chloromethane? Halogenated product is a haloalkane Naming: halogen atom is a substituent, replace –ine ending with –o 2/28/2021 -F fluoro Dr Seemal Jelani -Cl chloro Chem-160 -Br bromo -I iodo 20

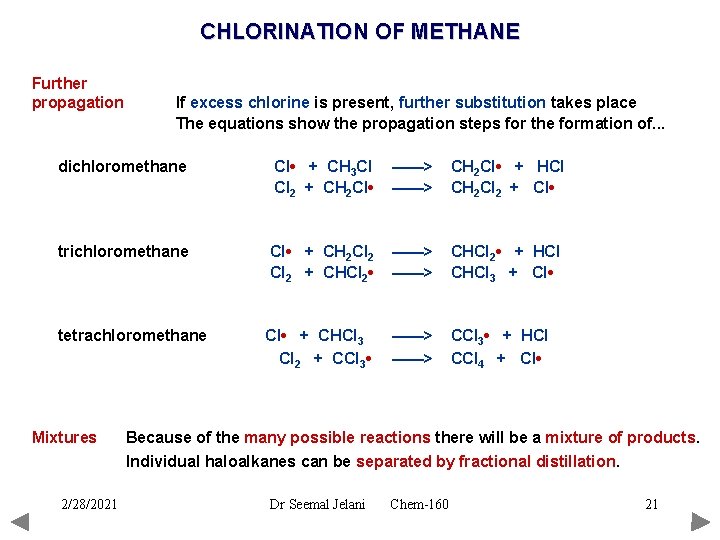
CHLORINATION OF METHANE Further propagation If excess chlorine is present, further substitution takes place The equations show the propagation steps for the formation of. . . dichloromethane Cl • + CH 3 Cl Cl 2 + CH 2 Cl • ——> CH 2 Cl • + HCl CH 2 Cl 2 + Cl • trichloromethane Cl • + CH 2 Cl 2 + CHCl 2 • ——> CHCl 2 • + HCl CHCl 3 + Cl • tetrachloromethane Cl • + CHCl 3 Cl 2 + CCl 3 • ——> CCl 3 • + HCl CCl 4 + Cl • Mixtures 2/28/2021 Because of the many possible reactions there will be a mixture of products. Individual haloalkanes can be separated by fractional distillation. Dr Seemal Jelani Chem-160 21

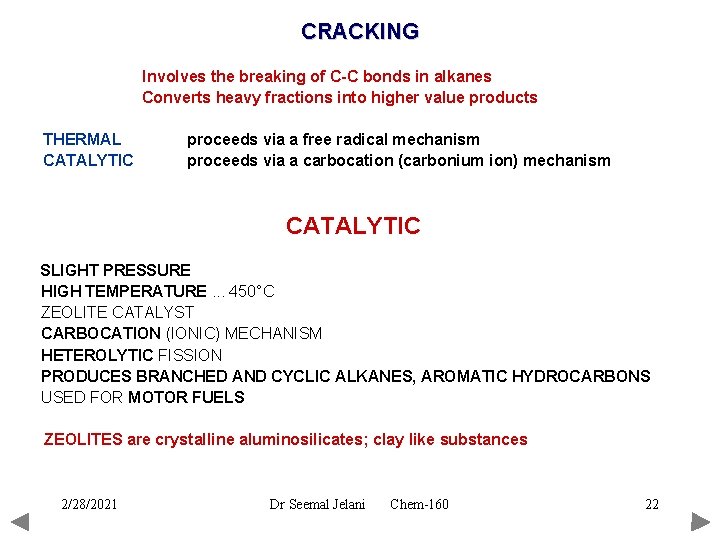
CRACKING Involves the breaking of C-C bonds in alkanes Converts heavy fractions into higher value products THERMAL CATALYTIC proceeds via a free radical mechanism proceeds via a carbocation (carbonium ion) mechanism CATALYTIC SLIGHT PRESSURE HIGH TEMPERATURE. . . 450°C ZEOLITE CATALYST CARBOCATION (IONIC) MECHANISM HETEROLYTIC FISSION PRODUCES BRANCHED AND CYCLIC ALKANES, AROMATIC HYDROCARBONS USED FOR MOTOR FUELS ZEOLITES are crystalline aluminosilicates; clay like substances 2/28/2021 Dr Seemal Jelani Chem-160 22

• At each level a mixture of compounds in a similar boiling range is taken off • Rough fractions can then be distilled further to obtain narrower boiling ranges • Some fractions are more important - usually the lower boiling point ones, high boiling fractions may be broken down into useful lower boiling ones - CRACKING 2/28/2021 Dr Seemal Jelani Chem-160 24

Sources of Alkanes • Natural gas – 90 to 95 percent methane – 5 to 10 percent ethane, and – a mixture of other low-boiling alkanes, chiefly propane, butane, and 2 methylpropane. • Petroleum – A thick liquid mixture of thousands of compounds, most of them hydrocarbons formed from the decomposition of marine plants and animals.