Alkaline Electrolysis Cells Materials Properties and Challenges Acknowledgements






![Measured conductivity of aqueous KOH Conductivity [S x cm-1] 3. 5 35 wt% KOH Measured conductivity of aqueous KOH Conductivity [S x cm-1] 3. 5 35 wt% KOH](https://slidetodoc.com/presentation_image_h2/e9ff30121b21d00ba5ccacf4e734e54e/image-21.jpg)
![Log differential intrusion [ m. L∙g -1] Characteristics of Porous Structure for Immobilization of Log differential intrusion [ m. L∙g -1] Characteristics of Porous Structure for Immobilization of](https://slidetodoc.com/presentation_image_h2/e9ff30121b21d00ba5ccacf4e734e54e/image-22.jpg)







- Slides: 49

Alkaline Electrolysis Cells: Materials, Properties and Challenges Acknowledgements to Mogens B. Mogensen Technical University of Denmark, DTU Risø Campus DK-4000 Roskilde Denmark colleagues at DTU Energy Conversion momo@dtu. dk 2 nd Joint European Summer School on Fuel Cell and Hydrogen Technology, Crete, September 25 th, 2012

Outline 1. Principles in alkaline water electrolysis 2. Commercial status 3. Materials in alkaline electrolysers a) Electrolyte b) Anode c) Cathode d) Separators, sealings, containments 4. Materials of electrolysers under development 5. Properties commercial systems – examples 6. Challenges – optimization of system 2 DTU Energy Conversion, Technical University of Denmark 25 October 2021

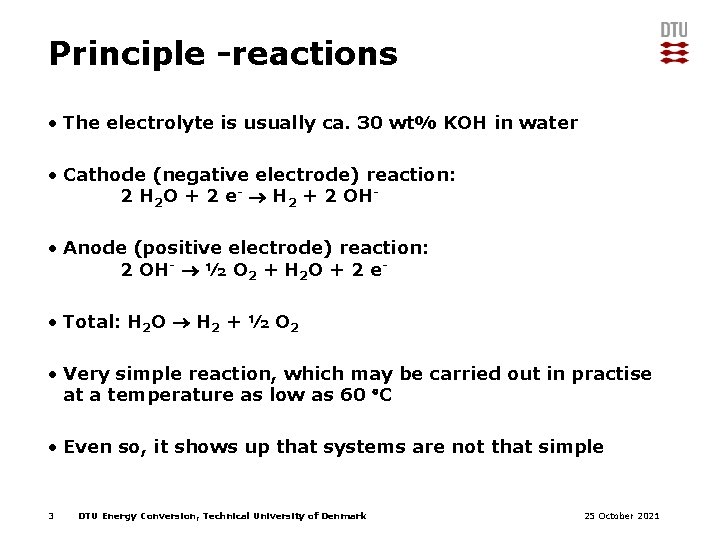
Principle -reactions • The electrolyte is usually ca. 30 wt% KOH in water • Cathode (negative electrode) reaction: 2 H 2 O + 2 e- H 2 + 2 OH • Anode (positive electrode) reaction: 2 OH- ½ O 2 + H 2 O + 2 e • Total: H 2 O H 2 + ½ O 2 • Very simple reaction, which may be carried out in practise at a temperature as low as 60 C • Even so, it shows up that systems are not that simple 3 DTU Energy Conversion, Technical University of Denmark 25 October 2021

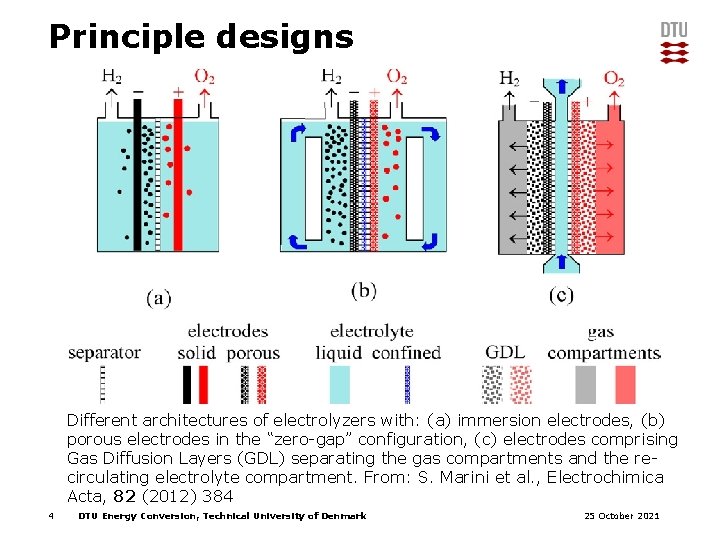
Principle designs Different architectures of electrolyzers with: (a) immersion electrodes, (b) porous electrodes in the “zero-gap” configuration, (c) electrodes comprising Gas Diffusion Layers (GDL) separating the gas compartments and the recirculating electrolyte compartment. From: S. Marini et al. , Electrochimica Acta, 82 (2012) 384 4 DTU Energy Conversion, Technical University of Denmark 25 October 2021

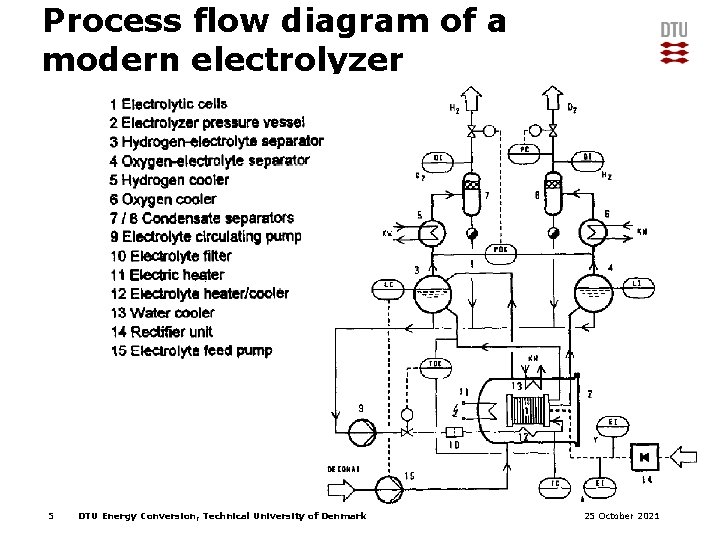
Process flow diagram of a modern electrolyzer 5 DTU Energy Conversion, Technical University of Denmark 25 October 2021

History of industrial water electrolysis Year Event 1800 Discovery of electrolytic splitting of water 1902 More than 400 industrial electrolyzers in operation 1939 First large electrolysis plant with capacity 10, 000 m 3 H 2 h-1 1948 First pressurized electrolyzer by Zdansky/Lonza 1966 First solid polymer electrolyte system (General Electric) 1972 Development of solid oxide water electrolysis started 1978 Development of advanced alkaline electrolysis started From: W. Kreuter and H. Hofmann, Int. J. Hydrogen Energy, 23, (1998) 661 6 DTU Energy Conversion, Technical University of Denmark 25 October 2021

Commercial alkaline electrolyzers • Norsk Hydro set up a small installation in Notodden in 1927, Norway, for test purposes, later followed by a large industrial installation in Rjukan, Norway where the energy was supplied by Vemork power station – the largest hydro power station in the world at that time. • The Norwegian company still exist with new owners, and today its name is NEL • Several other companies that produce alkaline electrolysers exist • Companies selling “big” systems, > 50 Nm 3 h-1: – NEL (NO) – Hydrogenics (BE, CA) – Linde (DE) – ELT (DE) – iht (CH) – Teledyn (USA) • Many more companies sell smaller systems 7 DTU Energy Conversion, Technical University of Denmark 25 October 2021

The alkaline electrolyser is commercial available Hans Jörg Fell, CTO 8 DTU Energy Conversion, Technical University of Denmark 25 October 2021

Photo of NEL on-site electrolyser The photo is from a presentation by NEL CTO Hans Jörg Fell, “Alkaline electrolysis for distributed and central hydrogen production”, International Water Electrolysis Symposium, Copenhagen, 10 -11 May. 2012. NEL does not tell the details of what is on their photos. As far as I can figure out by studying their homepage, http: //www. nel-hydrogen. com/home/, and various presentations, this is an atmospheric pressure, ca. 2. 2 MW, unit, which seems to be one of NEL’s units from which the bigger systems are built. The nominal production capacity is 500 Nm 3 H 2 h-1. 9 DTU Energy Conversion, Technical University of Denmark 25 October 2021

Hydrogenics alkaline electrolyser cell stack Photo from: Raymond Schmidt, Global Market Strategist, Hydrogenics, “Electrolysis for grid balancing”, International Water Electrolysis Symposium, Copenhagen, 10 -11 May. 2012. Hy. STAT® 10 – 10 10 DTU Energy Conversion, Technical University of Denmark 25 October 2021

Hydrogenics Alkaline system From Hydrogenics’ homepage: Hy. STAT® 10 – 10 10 Nm 3 H 2 h-1, 5. 4 k. Wh/Nm 3 H 2 DTU Energy Conversion, Technical University of Denmark

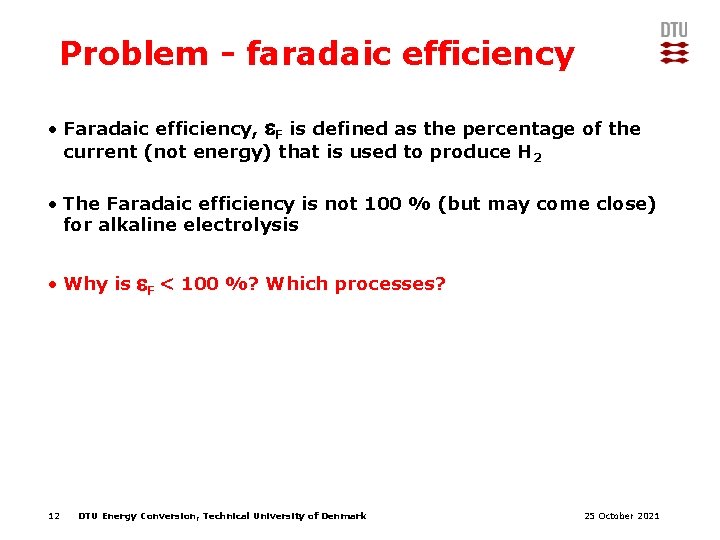
Problem - faradaic efficiency • Faradaic efficiency, F is defined as the percentage of the current (not energy) that is used to produce H 2 • The Faradaic efficiency is not 100 % (but may come close) for alkaline electrolysis • Why is 12 F < 100 %? Which processes? DTU Energy Conversion, Technical University of Denmark 25 October 2021

Materials in alkaline electrolysers • From the previous information it should be clear that a big number of components and even bigger number of materials are involved • It is not possible to cover all of them in this presentation, and honestly, I do not know which materials the commercial companies are using; I even do not know exactly which components each of them use. • Companies simple do not inform publicly about what they are doing. • No noble metals or other expensive materials! • Therefore, we can only know about what has been published from measurements in the laboratories 13 DTU Energy Conversion, Technical University of Denmark 25 October 2021

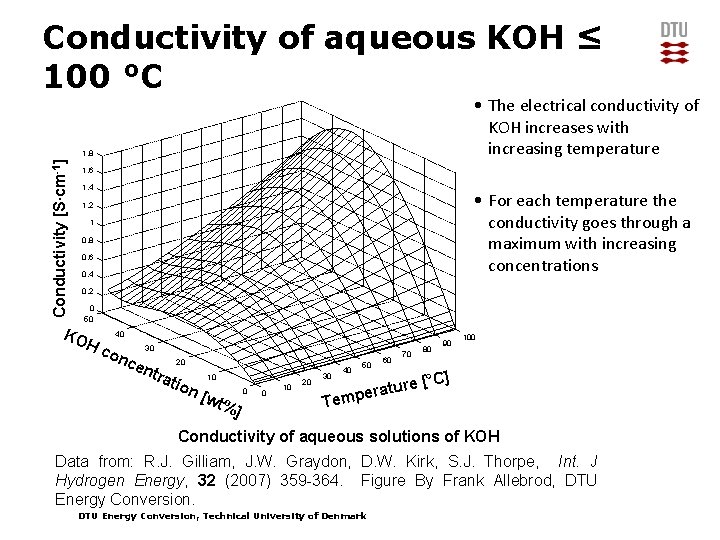
Electrolyte • The most used electrolyte for alkaline electrolysers is concentrated aqueous solution of KOH • Often 30 – 35 wt% KOH is used as this has the highest conductivity 14 DTU Energy Conversion, Technical University of Denmark 25 October 2021

Conductivity of aqueous KOH ≤ 100 °C • The electrical conductivity of KOH increases with increasing temperature Conductivity [S∙cm-1] 1. 8 1. 6 1. 4 • For each temperature the conductivity goes through a maximum with increasing concentrations 1. 2 1 0. 8 0. 6 0. 4 0. 2 0 50 KO 40 Hc on ce 30 ntr 20 atio n[ 10 wt% 0 ] 0 10 20 40 30 50 60 70 ture mpera 80 90 100 [°C] Te Conductivity of aqueous solutions of KOH Data from: R. J. Gilliam, J. W. Graydon, D. W. Kirk, S. J. Thorpe, Int. J Hydrogen Energy, 32 (2007) 359 -364. Figure By Frank Allebrod, DTU Energy Conversion, Technical University of Denmark

Questions • Why does the conductivity decrease above a certain concentration? • How are conductivity of electrolytes measured? • What size of conductivity is needed in electrochemical cells? DTU Energy Conversion, Technical University of Denmark

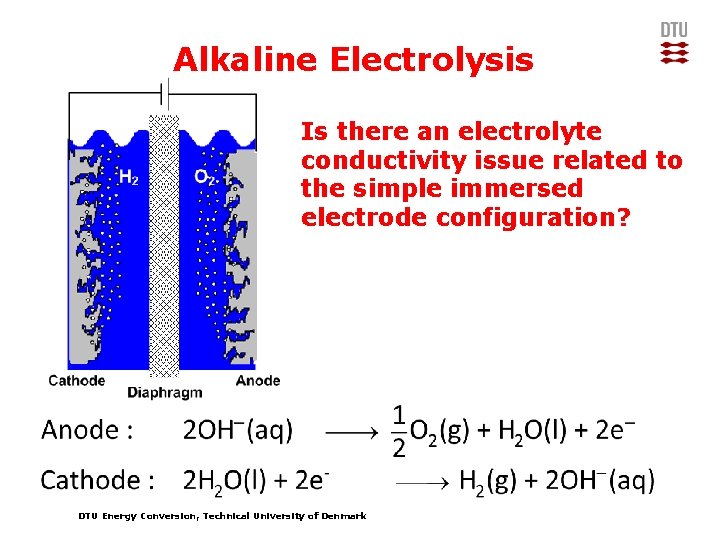
Alkaline Electrolysis Is there an electrolyte conductivity issue related to the simple immersed electrode configuration? DTU Energy Conversion, Technical University of Denmark

EMF - the equilibrium voltage Dependence of the reversible cell voltage Erev to the system pressure and the temperature for pure water at 1 bar (dash dot) and for aqueous KOH with a concentration of 45 wt% at a pressure of 1 bar (full line), 10 bar (dashed), 25 bar (o) and 50 bar (+). DTU Energy Conversion, Technical University of Denmark

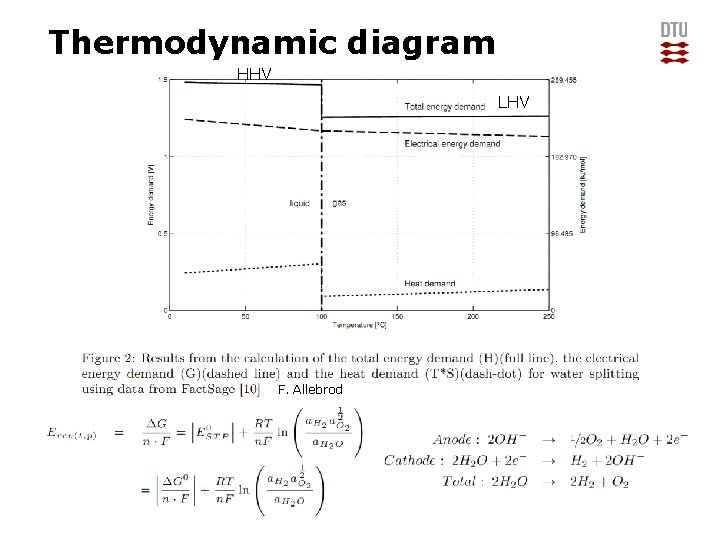
Thermodynamic diagram HHV LHV F. Allebrod DTU Energy Conversion, Technical University of Denmark

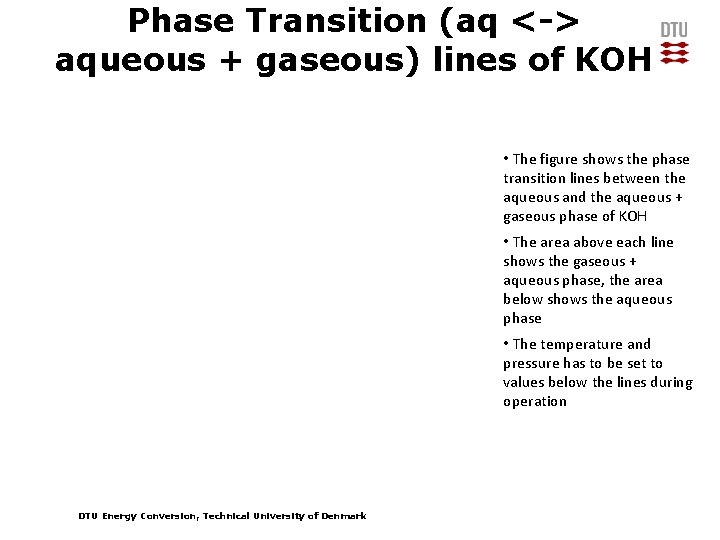
Phase Transition (aq <-> aqueous + gaseous) lines of KOH • The figure shows the phase transition lines between the aqueous and the aqueous + gaseous phase of KOH • The area above each line shows the gaseous + aqueous phase, the area below shows the aqueous phase • The temperature and pressure has to be set to values below the lines during operation DTU Energy Conversion, Technical University of Denmark
![Measured conductivity of aqueous KOH Conductivity S x cm1 3 5 35 wt KOH Measured conductivity of aqueous KOH Conductivity [S x cm-1] 3. 5 35 wt% KOH](https://slidetodoc.com/presentation_image_h2/e9ff30121b21d00ba5ccacf4e734e54e/image-21.jpg)
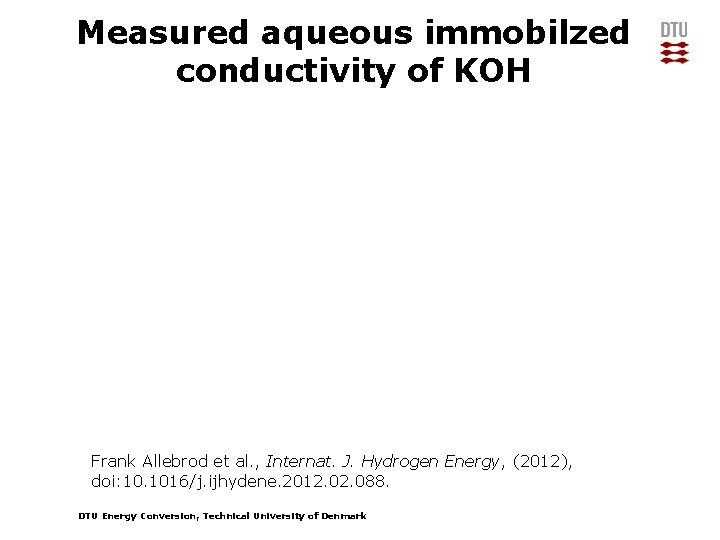
Measured conductivity of aqueous KOH Conductivity [S x cm-1] 3. 5 35 wt% KOH 3. 5 45 wt% KOH 3. 5 3 3 3 2. 5 2 2 2 1. 5 1 1 1 0. 5 0 0 100 200 0 300 0 measured literature values 100 200 Temperature [°C] 55 wt% KOH 0. 5 0 300 0 100 200 Frank Allebrod et al. , Internat. J. Hydrogen Energy, (2012), doi: 10. 1016/j. ijhydene. 2012. 088. DTU Energy Conversion, Technical University of Denmark 300
![Log differential intrusion m Lg 1 Characteristics of Porous Structure for Immobilization of Log differential intrusion [ m. L∙g -1] Characteristics of Porous Structure for Immobilization of](https://slidetodoc.com/presentation_image_h2/e9ff30121b21d00ba5ccacf4e734e54e/image-22.jpg)
Log differential intrusion [ m. L∙g -1] Characteristics of Porous Structure for Immobilization of Liquid KOH Solution 1. 4 1. 2 1 0. 8 0. 6 0. 4 0. 2 0 10 -3 -2 10 10 -1 Pore size [10 -6 m] 0 10 • Porosimetry analysis showed pore-sizes around 60 nm, as shown in the figure • The total porosity of the porous structure is about 51% Frank Allebrod et al. , Internat. J. Hydrogen Energy, (2012), doi: 10. 1016/j. ijhydene. 2012. 088. DTU Energy Conversion, Technical University of Denmark 1 10

Measured aqueous immobilzed conductivity of KOH Frank Allebrod et al. , Internat. J. Hydrogen Energy, (2012), doi: 10. 1016/j. ijhydene. 2012. 088. DTU Energy Conversion, Technical University of Denmark

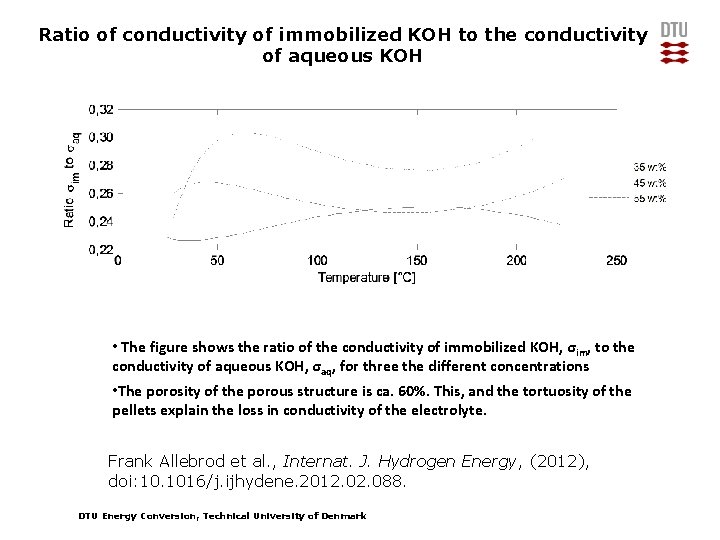
Ratio of conductivity of immobilized KOH to the conductivity of aqueous KOH • The figure shows the ratio of the conductivity of immobilized KOH, σ im, to the conductivity of aqueous KOH, σaq, for three the different concentrations • The porosity of the porous structure is ca. 60%. This, and the tortuosity of the pellets explain the loss in conductivity of the electrolyte. Frank Allebrod et al. , Internat. J. Hydrogen Energy, (2012), doi: 10. 1016/j. ijhydene. 2012. 088. DTU Energy Conversion, Technical University of Denmark

Comparison to commercial electrolytes/ diaphragms • A conductivity of immobilized KOH of 0. 25 S∙cm-1 and 0. 84 S∙cm-1 for 45 wt% was achieved at 80 C and 200 C, respectively • KOH immobilized in Zirfon, a commercially available diaphragm, was reported as 0. 6 S∙cm-1 at 80 C by Vermeiren et al. Unknown porosity. Zirfon is not stable at 200 C. P. Vermeiren, J. P. Moreels, A. Claes, H. Beckers, Electrode diaphragm electrode assembly for alkaline water electrolysers, Int J Hydrogen Energy. 34 (2009) 9305 -9315 DTU Energy Conversion, Technical University of Denmark

Conventional cathode • Raney nickel has been the most popular cathode materials since it was invented in 1948, but simple Ni sheets and Nicoated steel have also been used due to low cost • The name “Raney nickel” covers now a group of Ni alloys of Ni-Zn and Ni-Al. When the Raney Ni is treated in concentrated KOH then the Zn and the Al will be dissolved and a nano-porous Ni sponge is left behind. Due to the high surface area and the good electrocatalytic properties of Ni this forms a very good cathode = H 2 evoælution electrode 26 DTU Energy Conversion, Technical University of Denmark 25 October 2021

Cathodes recent developments • The composition of one of the best cathodes was: 70% Mo -doped Raney Ni (A 7000), 10% Mo. O 3, 5% Cu, 5% graphite and 10% PTFE. • It is known that 1%, or less, of Mo in Raney Ni improves stability and HER activity of this catalyst, and that even coarse mixtures of Ni alloys and molybdenites may perform better than the individual catalysts in HER DTU Energy Conversion, Technical University of Denmark

Anodes • Ni and Ag-coated Ni • Activated Nickel electrodes (Ni-Co, Mo, Pt) DTU Energy Conversion, Technical University of Denmark

Separator, sealing, containments • Separator was originally made from asbestos, but this is now forbidden due to health risk • Now, other materials like Zirfon (Zr. O 2 with polymer binder) or Ni. O have been used. • The sealing may be PTFE or similar stable polymers metal-ceramic-metal layers for higher temperature • Containment is probably Ni-coated steel in most cases • It is difficult to get info about what industry actually use DTU Energy Conversion, Technical University of Denmark

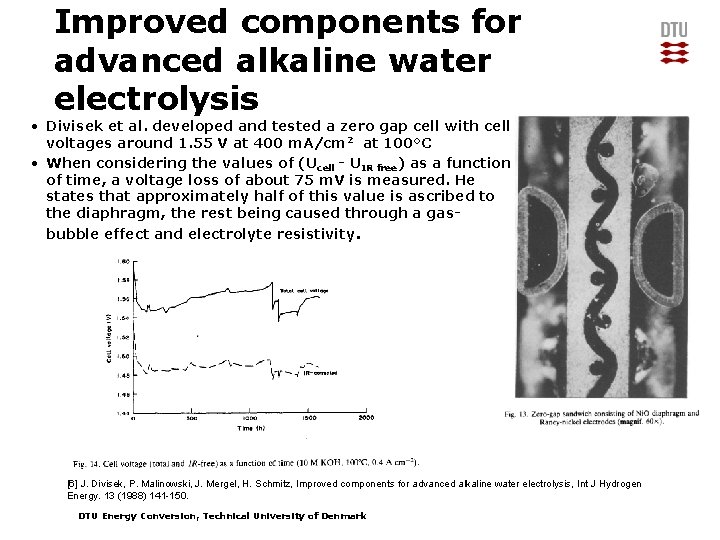
Improved components for advanced alkaline water electrolysis • Divisek et al. developed and tested a zero gap cell with cell voltages around 1. 55 V at 400 m. A/cm 2 at 100°C • When considering the values of (Ucell - UIR free) as a function of time, a voltage loss of about 75 m. V is measured. He states that approximately half of this value is ascribed to the diaphragm, the rest being caused through a gasbubble effect and electrolyte resistivity. [6] J. Divisek, P. Malinowski, J. Mergel, H. Schmitz, Improved components for advanced alkaline water electrolysis, Int J Hydrogen Energy. 13 (1988) 141 -150. DTU Energy Conversion, Technical University of Denmark

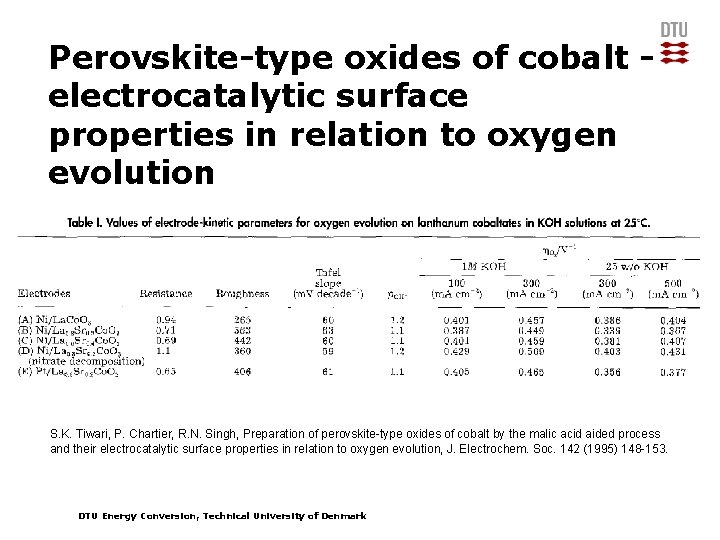
Perovskite-type oxides of cobalt electrocatalytic surface properties in relation to oxygen evolution S. K. Tiwari, P. Chartier, R. N. Singh, Preparation of perovskite-type oxides of cobalt by the malic acid aided process and their electrocatalytic surface properties in relation to oxygen evolution, J. Electrochem. Soc. 142 (1995) 148 -153. DTU Energy Conversion, Technical University of Denmark

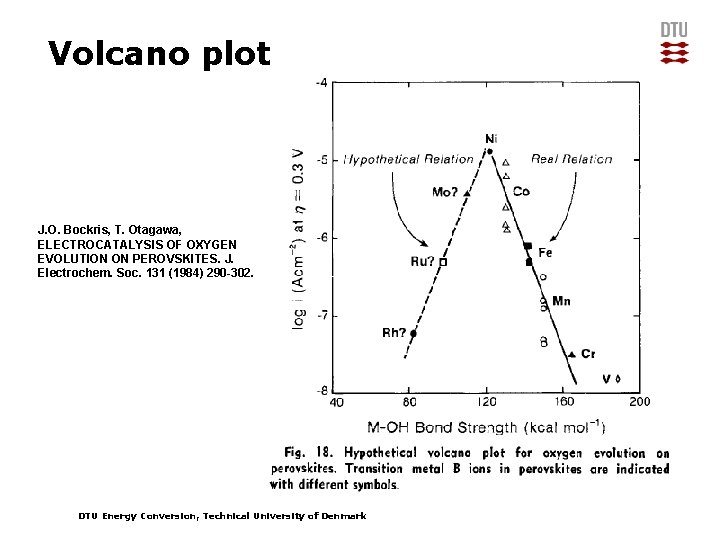
Volcano plot J. O. Bockris, T. Otagawa, ELECTROCATALYSIS OF OXYGEN EVOLUTION ON PEROVSKITES. J. Electrochem. Soc. 131 (1984) 290 -302. DTU Energy Conversion, Technical University of Denmark

Tentative conclusion on anode materials • After reviewing a number of paper about catalysts for the OER in alkaline media, it was found that Co 3 O 4, Raney-Nickel, Ru. O 2 and Ir. O 2 are most active. • Never the less, it has also been shown that the difference to perovskite-type materials are rather small. It is likely that, towards higher current densities, other attributes like cell design, diffusion and gas bubbles are more important DTU Energy Conversion, Technical University of Denmark

New developments • It is well known that there will be important advantages if the operation temperature could be raised from the 60 – 120 C to say 200 – 300 C • Which advantages could you think of? • Naturally, there would also be disadvantages. • Which could you think of? • Inspiration may be taken from the alkaline fuel cell 34 DTU Energy Conversion, Technical University of Denmark 25 October 2021

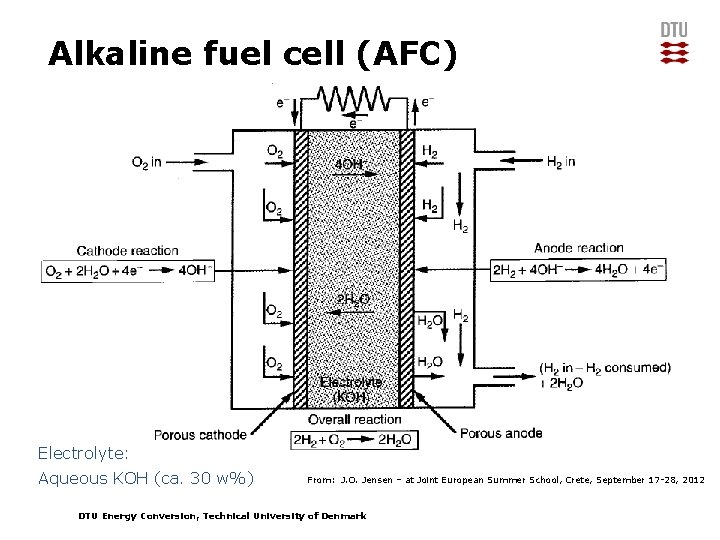
Alkaline fuel cell (AFC) Electrolyte: Aqueous KOH (ca. 30 w%) From: J. O. Jensen – at Joint European Summer School, Crete, September 17 -28, 2012 DTU Energy Conversion, Technical University of Denmark

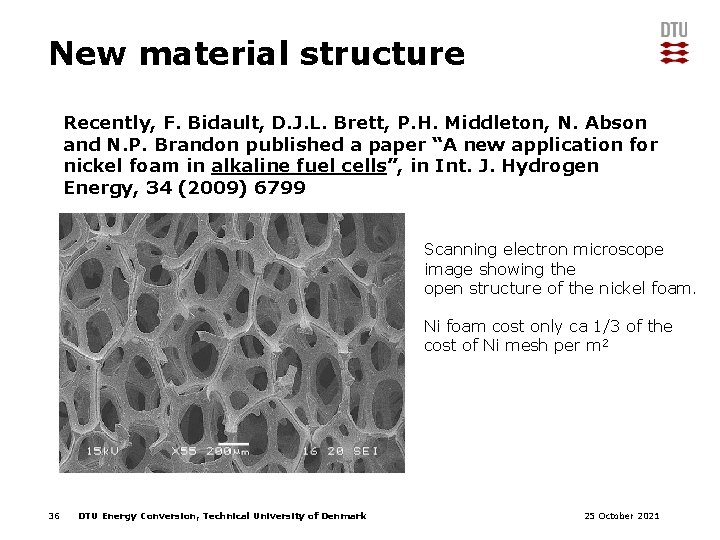
New material structure Recently, F. Bidault, D. J. L. Brett, P. H. Middleton, N. Abson and N. P. Brandon published a paper “A new application for nickel foam in alkaline fuel cells”, in Int. J. Hydrogen Energy, 34 (2009) 6799 Scanning electron microscope image showing the open structure of the nickel foam. Ni foam cost only ca 1/3 of the cost of Ni mesh per m 2 36 DTU Energy Conversion, Technical University of Denmark 25 October 2021

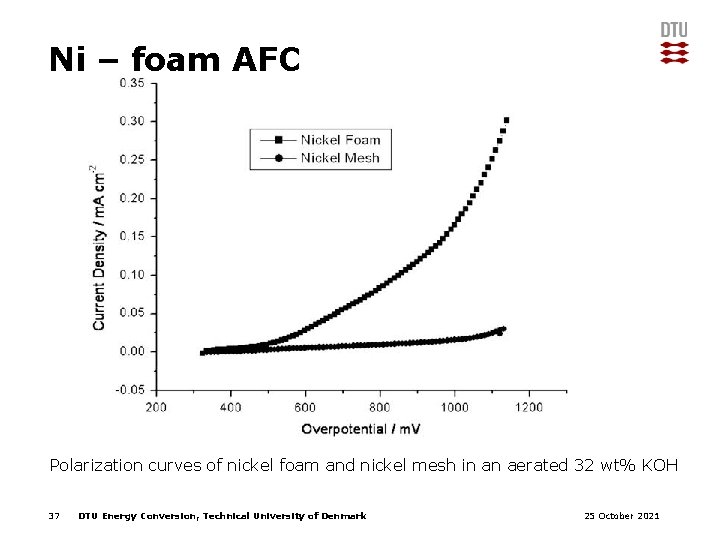
Ni – foam AFC Polarization curves of nickel foam and nickel mesh in an aerated 32 wt% KOH 37 DTU Energy Conversion, Technical University of Denmark 25 October 2021

Pressure and temperature • It is well established that both pressure and temperature increase the electrode kinetics – for both oxygen and hydrogen electrode • So what to do if we should really optimize? • As high temperature and pressure as possible! • Materials put limits, however! 38 DTU Energy Conversion, Technical University of Denmark 25 October 2021

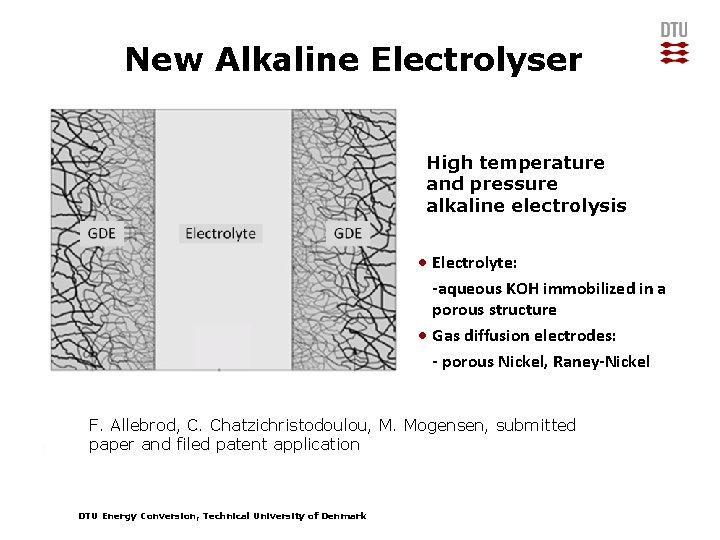
New Alkaline Electrolyser High temperature and pressure alkaline electrolysis • Electrolyte: -aqueous KOH immobilized in a porous structure • Gas diffusion electrodes: - porous Nickel, Raney-Nickel F. Allebrod, C. Chatzichristodoulou, M. Mogensen, submitted paper and filed patent application DTU Energy Conversion, Technical University of Denmark

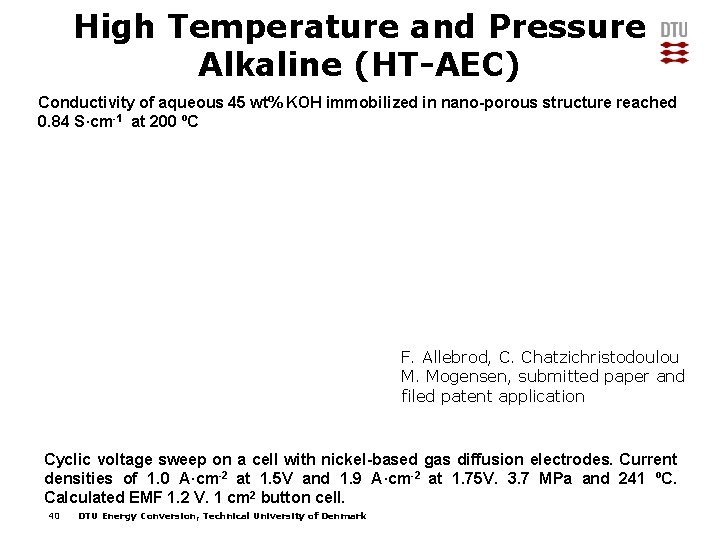
High Temperature and Pressure Alkaline (HT-AEC) Conductivity of aqueous 45 wt% KOH immobilized in nano-porous structure reached 0. 84 S·cm-1 at 200 ºC F. Allebrod, C. Chatzichristodoulou M. Mogensen, submitted paper and filed patent application Cyclic voltage sweep on a cell with nickel-based gas diffusion electrodes. Current densities of 1. 0 A·cm-2 at 1. 5 V and 1. 9 A·cm-2 at 1. 75 V. 3. 7 MPa and 241 ºC. Calculated EMF 1. 2 V. 1 cm 2 button cell. 40 DTU Energy Conversion, Technical University of Denmark

SEM analysis of the used foam and the Cell surface • The electrolysis cells are pressed out of Nickel and Inconel foams with a pore size of 200 -450 µm as delivered • After the press and sintering production method the pore size is reduced to 50 -150 µm F. Allebrod et al. DTU Energy Conversion, Technical University of Denmark

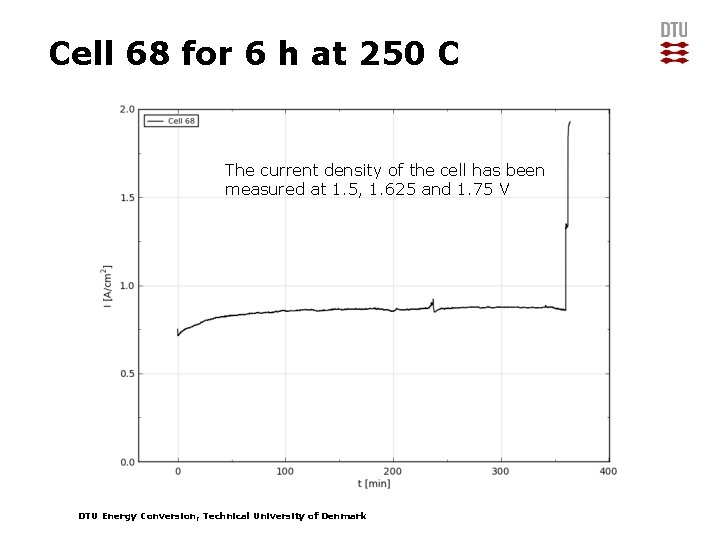
Cell 68 for 6 h at 250 C The current density of the cell has been measured at 1. 5, 1. 625 and 1. 75 V DTU Energy Conversion, Technical University of Denmark

Performance of commercial Alkaline Electrolysers • Laboratory results are fine, in particular for natural and technical science • Commercial field results are better for economy considerations 43 DTU Energy Conversion, Technical University of Denmark 25 October 2021

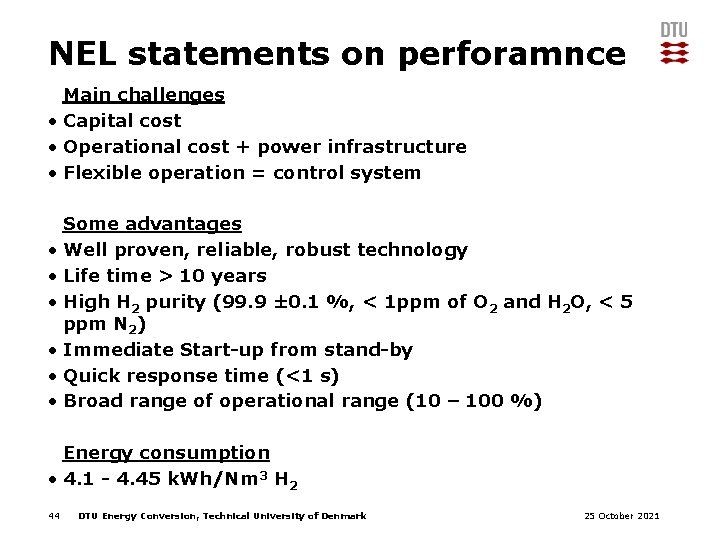
NEL statements on perforamnce Main challenges • Capital cost • Operational cost + power infrastructure • Flexible operation = control system Some advantages • Well proven, reliable, robust technology • Life time > 10 years • High H 2 purity (99. 9 ± 0. 1 %, < 1 ppm of O 2 and H 2 O, < 5 ppm N 2) • Immediate Start-up from stand-by • Quick response time (<1 s) • Broad range of operational range (10 – 100 %) Energy consumption • 4. 1 - 4. 45 k. Wh/Nm 3 H 2 44 DTU Energy Conversion, Technical University of Denmark 25 October 2021

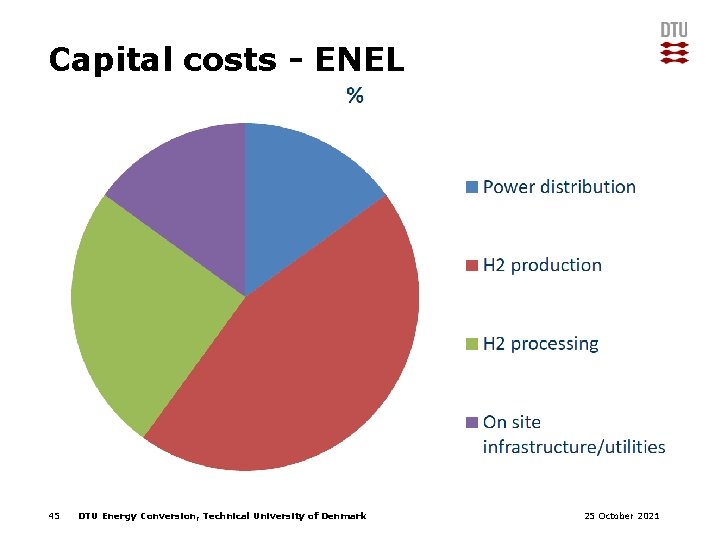
Capital costs - ENEL 45 DTU Energy Conversion, Technical University of Denmark 25 October 2021

Operational costs -ENEL • Electricity: 98% • Remaining 2% : – Cooling water make-up – Operation & maintenance – Electrolyte charge + make-up – Raw water – Purification – Emergency (N 2, diesel back-up) 46 DTU Energy Conversion, Technical University of Denmark 25 October 2021

Hydrogenics Hy. STAT® - Best Cell Stack Performance • Alkaline electrolysis: 30% vol. KOH • Pressure: 10 barg to 30 bar (barg = gauge pressure, i. e. , pressure in bars above ambient pressure) • Conversion efficiency: 4. 44 k. Wh/Nm 3 H 2 (HHV: 80%, LHV: 68%) • Lifetime: 60, 000 hours (6. 8 years) • Hydrogen purity: 99. 9 % – < 1000 ppm O 2 in H 2 – 12 ppm N 2 – H 2 O saturated From: Raymond Schmidt, Global Market Strategist, Hydrogenics, “Electrolysis for grid balancing”, International Water Electrolysis Symposium, Copenhagen, 10 -11 May. 2012. 47 DTU Energy Conversion, Technical University of Denmark 25 October 2021

Challenges • At the end it is all about economy • This is first of all electricity cost and next, production rate, efficiency plus investment cost • This could easily be an economic lesson of its own, but as this school is not about economy let us spend the remaining time for discussion of efficiency 48 DTU Energy Conversion, Technical University of Denmark 25 October 2021

Please find the “round trip” efficiency of H 2 – O 2 Alkaline Electrolyser – Fuel Cell • From electricity out of the grid back into the grid? • Let us discuss and find the numbers (Google) together. First which numbers do you need? • Are there other relevant efficiencies? 49 DTU Energy Conversion, Technical University of Denmark 25 October 2021