Alicyclic Compounds Compounds which contain closed rings comprised



- Slides: 13

Alicyclic Compounds

Compounds which contain closed rings comprised only of carbon atoms are known collectively as carbocyclic or homocyclic compounds such as benzene, naphthalene, and anthracene. Heterocyclic compounds Alicyclic compounds are called also aliphatic cyclic compounds, they have properties similar to aliphatic compounds in addition to the very special properties due to cyclic nature. The saturated alicyclic hydrocarbons have the general formula Cn. H 2 n (the same as that of the alkenes). General formula of saturated alicyclic hydrocarbons. ** For one ring ** For two rings ** For three rings Cn. H 2 n-2 Cn. H 2 n-4

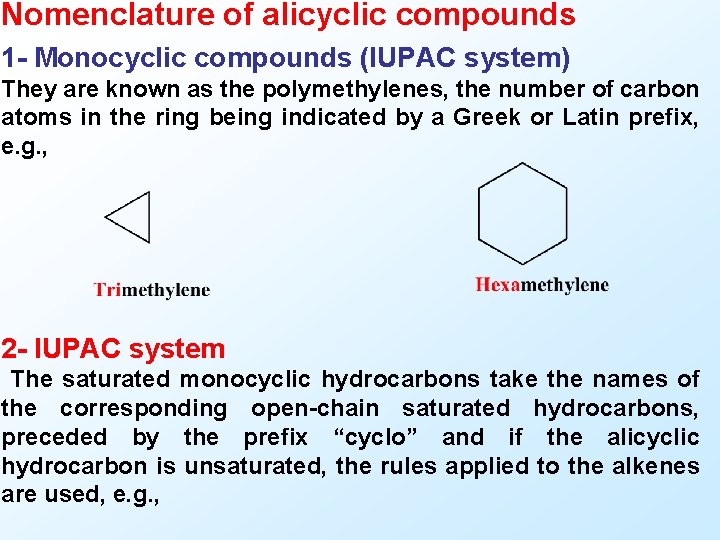
Nomenclature of alicyclic compounds 1 - Monocyclic compounds (IUPAC system) They are known as the polymethylenes, the number of carbon atoms in the ring being indicated by a Greek or Latin prefix, e. g. , 2 - IUPAC system The saturated monocyclic hydrocarbons take the names of the corresponding open-chain saturated hydrocarbons, preceded by the prefix “cyclo” and if the alicyclic hydrocarbon is unsaturated, the rules applied to the alkenes are used, e. g. ,

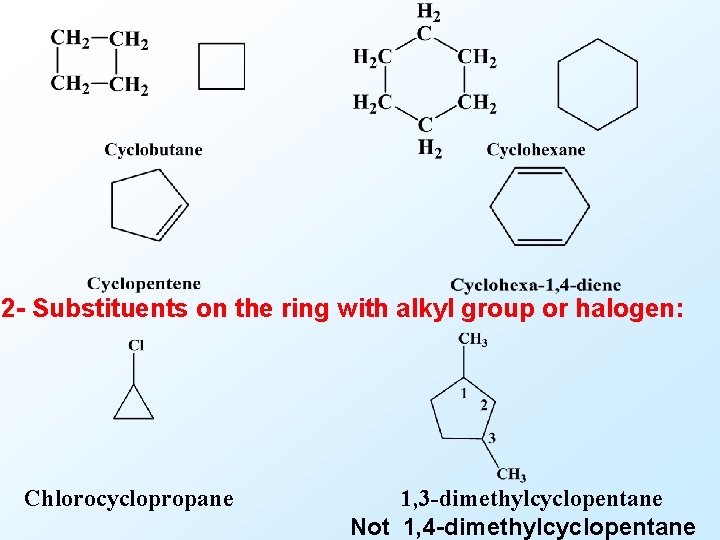
2 - Substituents on the ring with alkyl group or halogen: Chlorocyclopropane 1, 3 -dimethylcyclopentane Not 1, 4 -dimethylcyclopentane

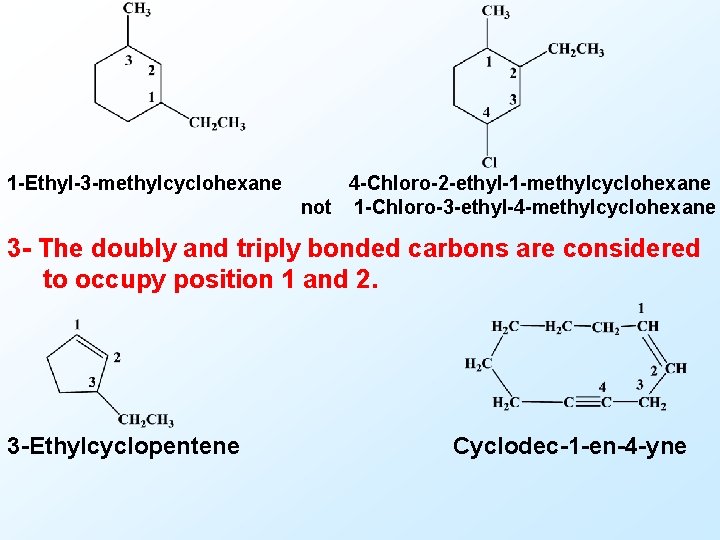
1 -Ethyl-3 -methylcyclohexane 4 -Chloro-2 -ethyl-1 -methylcyclohexane not 1 -Chloro-3 -ethyl-4 -methylcyclohexane 3 - The doubly and triply bonded carbons are considered to occupy position 1 and 2. 3 -Ethylcyclopentene Cyclodec-1 -en-4 -yne

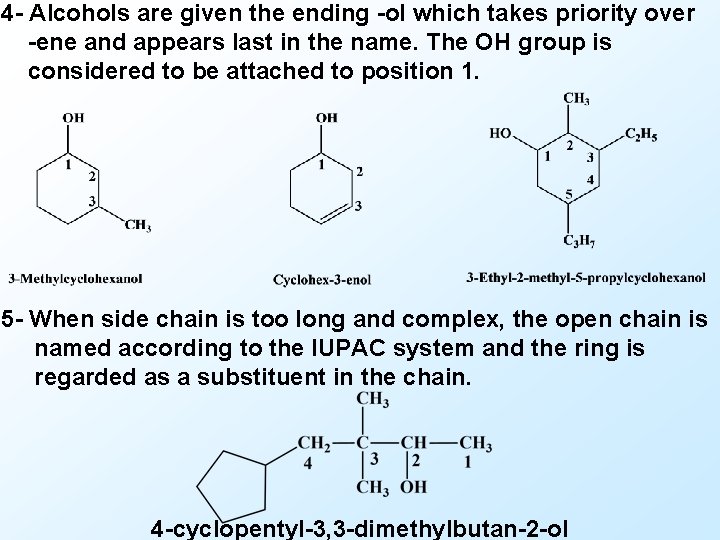
4 - Alcohols are given the ending -ol which takes priority over -ene and appears last in the name. The OH group is considered to be attached to position 1. 5 - When side chain is too long and complex, the open chain is named according to the IUPAC system and the ring is regarded as a substituent in the chain. 4 -cyclopentyl-3, 3 -dimethylbutan-2 -ol

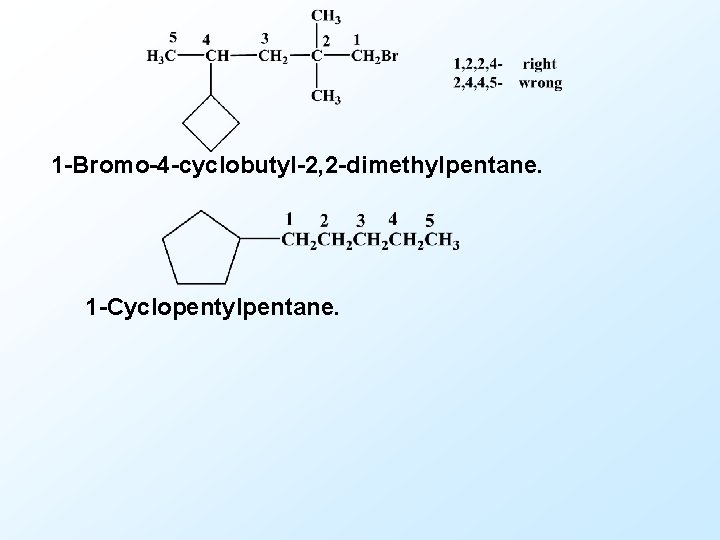
1 -Bromo-4 -cyclobutyl-2, 2 -dimethylpentane. 1 -Cyclopentylpentane.

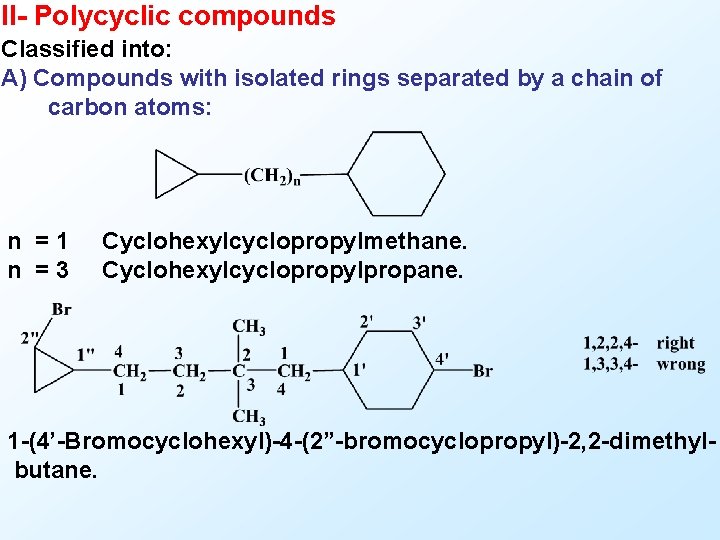
II- Polycyclic compounds Classified into: A) Compounds with isolated rings separated by a chain of carbon atoms: n =1 n =3 Cyclohexylcyclopropylmethane. Cyclohexylcyclopropylpropane. 1 -(4’-Bromocyclohexyl)-4 -(2”-bromocyclopropyl)-2, 2 -dimethylbutane.

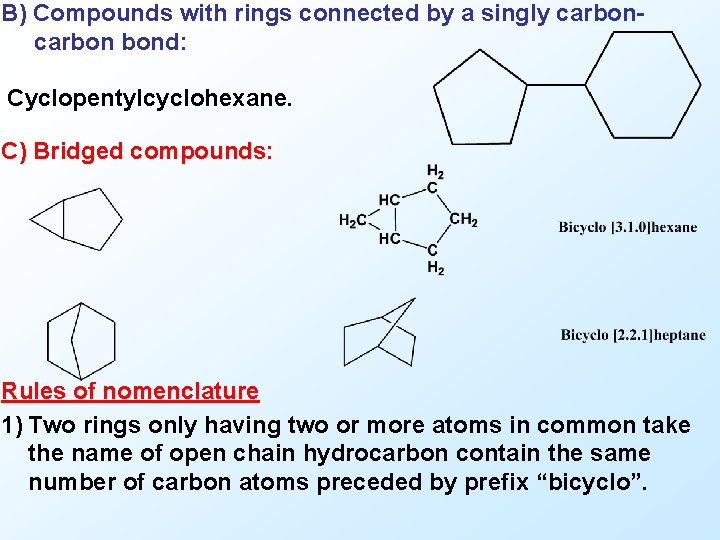
B) Compounds with rings connected by a singly carbon bond: Cyclopentylcyclohexane. C) Bridged compounds: Rules of nomenclature 1) Two rings only having two or more atoms in common take the name of open chain hydrocarbon contain the same number of carbon atoms preceded by prefix “bicyclo”.

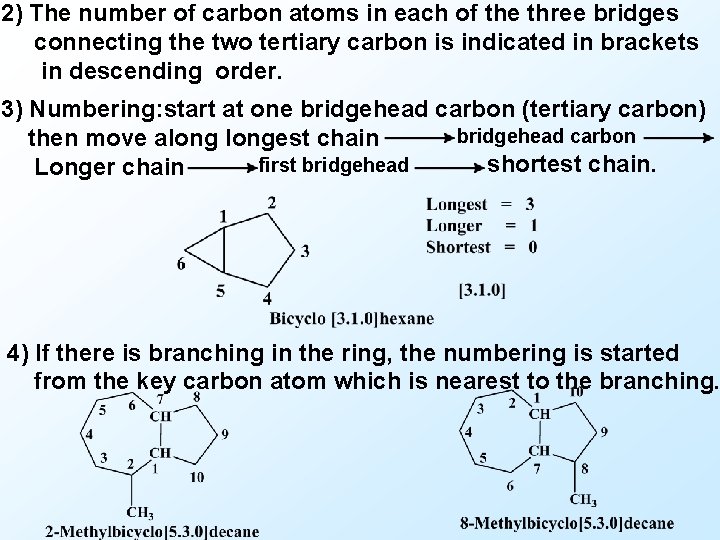
2) The number of carbon atoms in each of the three bridges connecting the two tertiary carbon is indicated in brackets in descending order. 3) Numbering: start at one bridgehead carbon (tertiary carbon) bridgehead carbon then move alongest chain shortest chain. first bridgehead Longer chain 4) If there is branching in the ring, the numbering is started from the key carbon atom which is nearest to the branching.

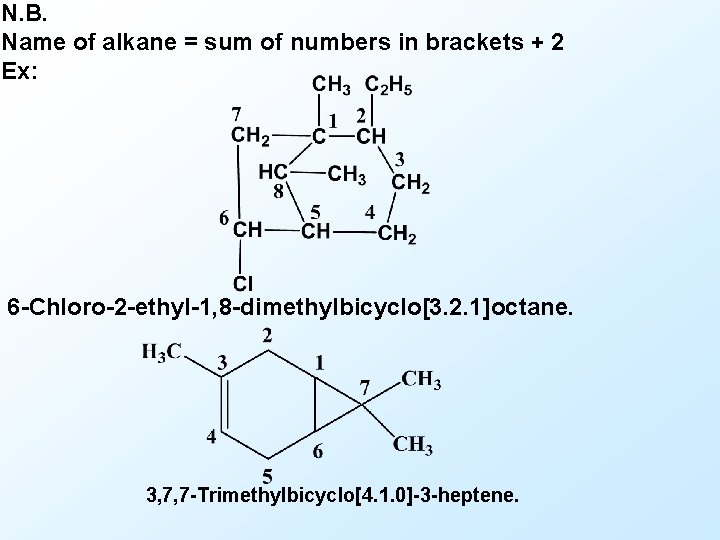
N. B. Name of alkane = sum of numbers in brackets + 2 Ex: 6 -Chloro-2 -ethyl-1, 8 -dimethylbicyclo[3. 2. 1]octane. 3, 7, 7 -Trimethylbicyclo[4. 1. 0]-3 -heptene.

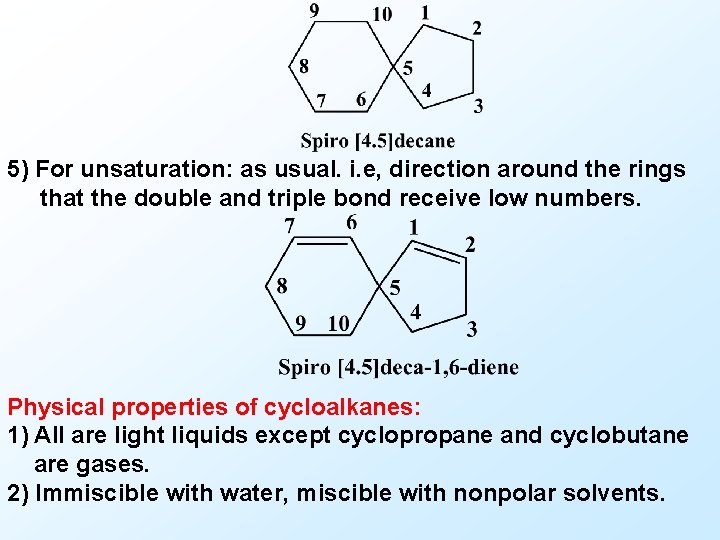
D) Spiro compounds Rules of nomenclature: 1) Spiro compounds consists of only two alicyclic rings having one common atom. They are named by placing “spiro” before the name of the normal acyclic hydrocarbon of the same total number of carbon atoms. 2) The number of carbon atoms linked to the spiro carbon atom in each ring is indicated in ascending order in brackets between the spiro prefix and the name of hydrocarbon. 3) Name: sum of no. in brackets + 1. 4) Numbering: start from carbon atom next to spiro atom through the smaller ring and then through the spiro atom and around the second larger ring.

5) For unsaturation: as usual. i. e, direction around the rings that the double and triple bond receive low numbers. Physical properties of cycloalkanes: 1) All are light liquids except cyclopropane and cyclobutane are gases. 2) Immiscible with water, miscible with nonpolar solvents.