ALDEHYDES AND KETONES CO Bond Ketones and Aldehydes

ALDEHYDES AND KETONES
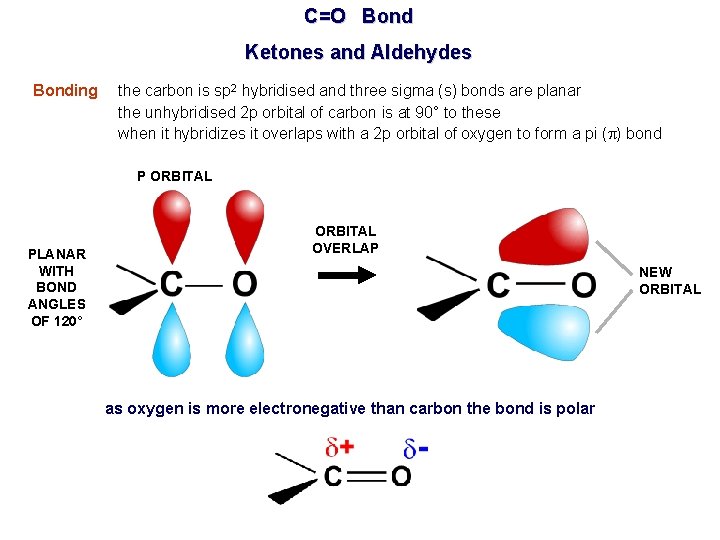
C=O Bond Ketones and Aldehydes Bonding the carbon is sp 2 hybridised and three sigma (s) bonds are planar the unhybridised 2 p orbital of carbon is at 90° to these when it hybridizes it overlaps with a 2 p orbital of oxygen to form a pi (p) bond P ORBITAL PLANAR WITH BOND ANGLES OF 120° ORBITAL OVERLAP NEW ORBITAL as oxygen is more electronegative than carbon the bond is polar
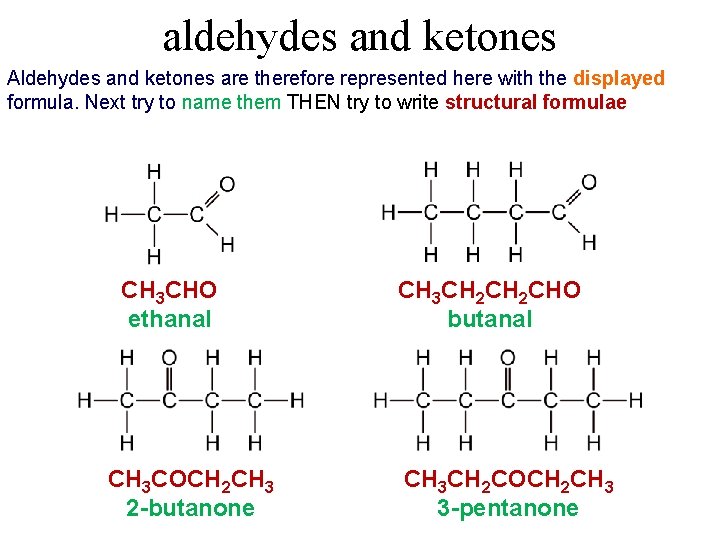
aldehydes and ketones Aldehydes and ketones are therefore represented here with the displayed formula. Next try to name them THEN try to write structural formulae CH 3 CHO ethanal CH 3 COCH 2 CH 3 2 -butanone CH 3 CH 2 CHO butanal CH 3 CH 2 COCH 2 CH 3 3 -pentanone

Nomenclature for aldehydes • IUPAC rules: • Select as the parent chain the longest continuous chain that has the carbonyl • Changing the corresponding alkane name (ending with “e”) to an ending with “al” Propanal 4 -Methylpentanal 2 -Ethylpentanal
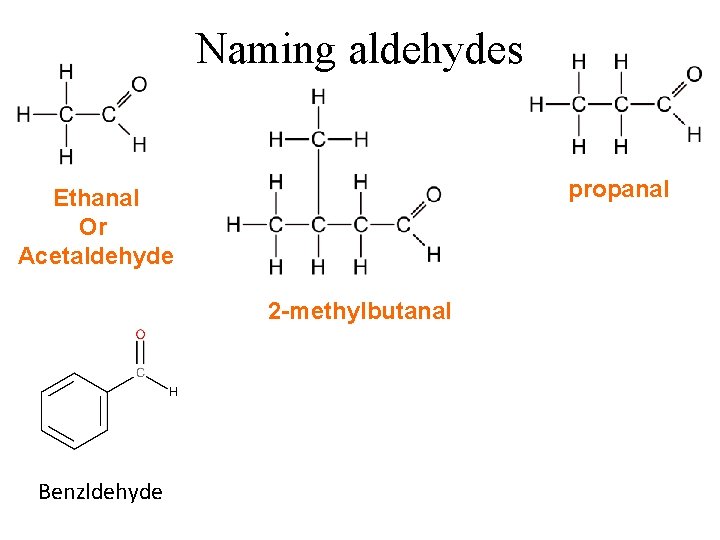
Naming aldehydes propanal Ethanal Or Acetaldehyde 2 -methylbutanal Benzldehyde

Nomenclature for ketones • • IUPAC: Select as the parent chain the longest continuous chain that has the carbonyl carbon Name the parent chain by removing the “e” and adding “one” Number the chain to give the carbonyl group the lowest numbering.
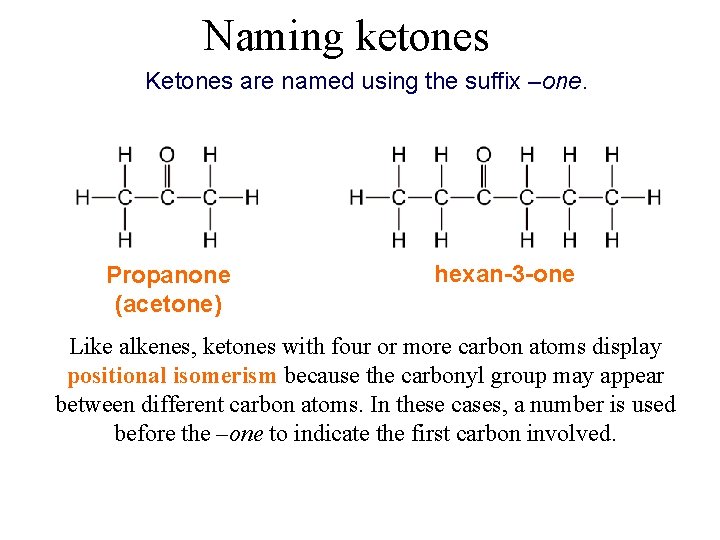
Naming ketones Ketones are named using the suffix –one. Propanone (acetone) hexan-3 -one Like alkenes, ketones with four or more carbon atoms display positional isomerism because the carbonyl group may appear between different carbon atoms. In these cases, a number is used before the –one to indicate the first carbon involved.
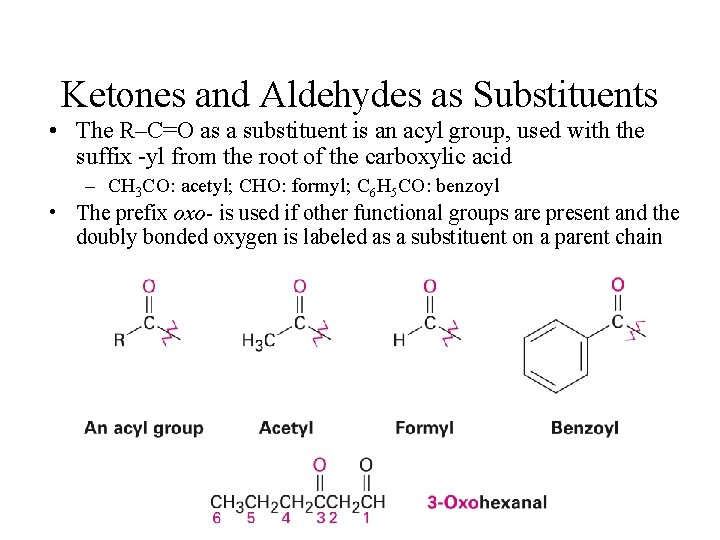
Ketones and Aldehydes as Substituents • The R–C=O as a substituent is an acyl group, used with the suffix -yl from the root of the carboxylic acid – CH 3 CO: acetyl; CHO: formyl; C 6 H 5 CO: benzoyl • The prefix oxo- is used if other functional groups are present and the doubly bonded oxygen is labeled as a substituent on a parent chain

Natural aldehydes and ketones • A wide variety of biologically relevant molecules possess aldehyde and/or ketone functional groups:

Naming Ketones Practice
Answers

CARBONYL COMPOUNDS - FORMULAE Aldehyde Ketone Molecular C 3 H 6 O Structural C 2 H 5 CHO C 2 H 5 CH 3 COCH 3 C=O H Displayed H C 3 H 6 O Both have the molecular formula C=O CH 3 H H H C C C H H Skeletal O O H H O H C C C H H O H
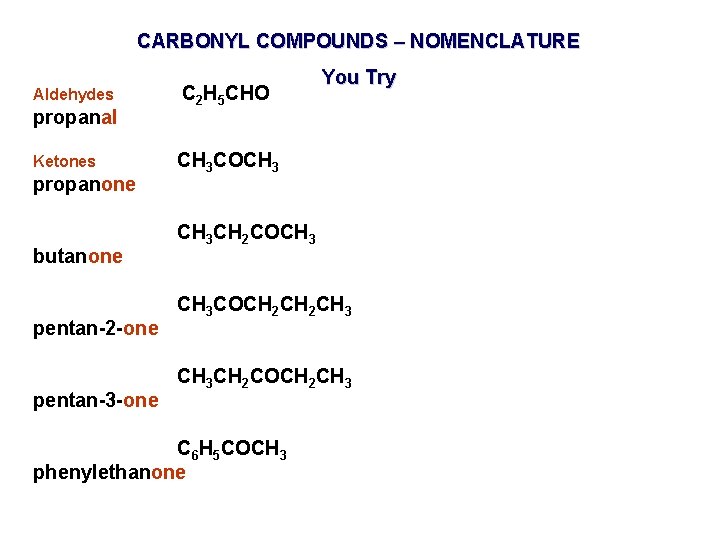
CARBONYL COMPOUNDS – NOMENCLATURE Aldehydes propanal Ketones propanone butanone pentan-2 -one pentan-3 -one C 2 H 5 CHO You Try CH 3 COCH 3 CH 2 COCH 3 COCH 2 CH 3 CH 2 COCH 2 CH 3 C 6 H 5 COCH 3 phenylethanone
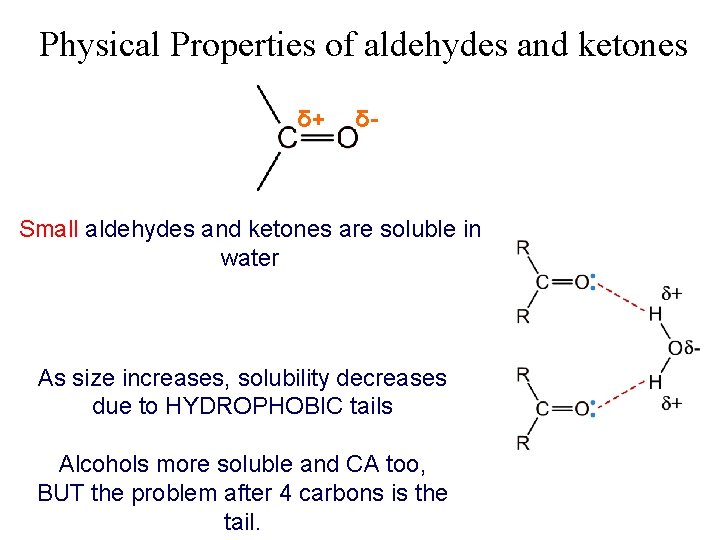
Physical Properties of aldehydes and ketones δ+ δ- Small aldehydes and ketones are soluble in water As size increases, solubility decreases due to HYDROPHOBIC tails Alcohols more soluble and CA too, BUT the problem after 4 carbons is the tail.
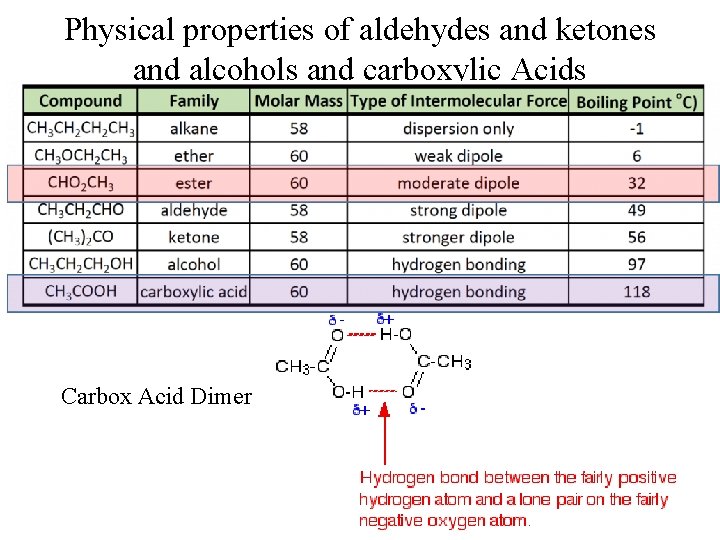
Physical properties of aldehydes and ketones and alcohols and carboxylic Acids Carbox Acid Dimer

Summary of Boiling Points The general increase in boiling points from alkanes to aldehydes/ketones and alcohols is due to the intermolecular forces l Alkanes are only held together by van der Waals forces. These forces increase with the size/length of a molecule. Ketones and Aldehydes polar carbonyl means they have van der Waals forces and dipole–dipole interactions. l Alcohols have van der Waals forces and dipole–dipole forces and hydrogen bonds l Carboxylic acids have 2 times the hydrogen bonding and have the highest of all the boiling points l

REACTIONS

Oxidation/Reduction Reactions • Commonly Termed ‘REDOX’ Reactions • From General Chemistry, we Will Recall Ø Oxidation: Loss of Electrons Ø Reduction: Gain of Electrons • Organic Chemists will Typically use Different Definitions Ø Reduction: Increase Hydrogen ØOxidation: Increase Oxygen •

Preparation of aldehydes and ketones • Primary (1 o) alcohols are oxidized to aldehydes (and subsequently to carboxylic acids) • Secondary (2 o) alcohols are oxidized to ketones [O] = acidified KMn. O 4 or K 2 Cr 2 O 7

Oxidation and reduction of aldehydes and ketones Oxidation reactions • Aldehydes can be oxidized easily to carboxylic acids • Ketones are resistant to oxidation.

3 Reactions Types of aldehydes and ketones: 1) Oxidation: Aldehydes to Carboxylic Acids (C. A. ) Ketones Het 2) Reduction: Aldehydes 10 Alcohols and Ketone 2 o Alcohols 3) Nucleophilic addition: Aldehydes are more reactive than Ketones

alkane alcohol reduction aldehyde ketone oxidation carboxylic acid addition product nucleophilic addition

nucleophilic addition to carbonyl:
![: Nu Addition, [O] and reduction Carbonyls The chemical properties of aldehydes and ketones : Nu Addition, [O] and reduction Carbonyls The chemical properties of aldehydes and ketones](http://slidetodoc.com/presentation_image/372e8b82a079df6b9684d47995da3681/image-24.jpg)
: Nu Addition, [O] and reduction Carbonyls The chemical properties of aldehydes and ketones result from the polar nature of the C=O bond. δ+ l The positive charge on the carbon nucleophiles δ- atom is attacked by : CNCYNAIDE IS DEADLY l l l Aldehydes and ketones can be reduced, forming primary and secondary alcohols respectively. Aldehydes may also be oxidized to carboxylic acids. We can alsohave : Nu addition (both are added)
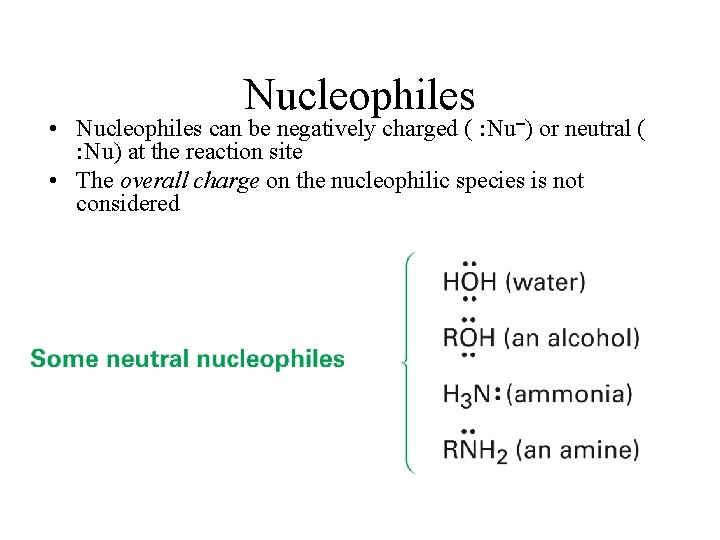
Nucleophiles • Nucleophiles can be negatively charged ( : Nu ) or neutral ( : Nu) at the reaction site • The overall charge on the nucleophilic species is not considered
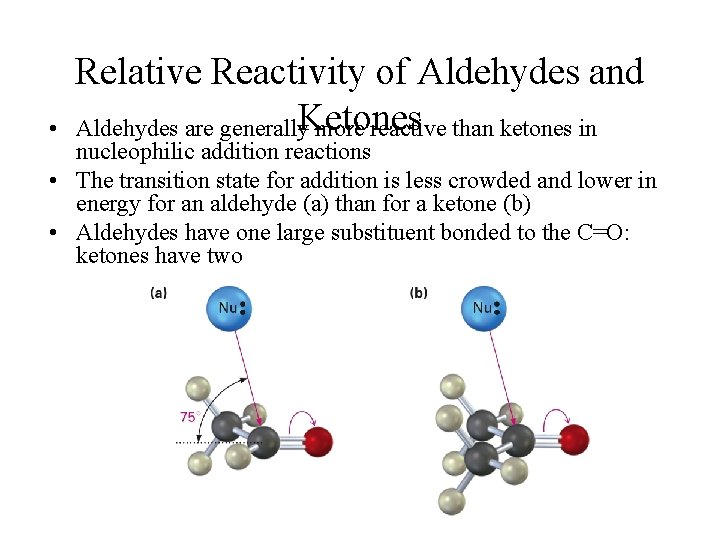
• Relative Reactivity of Aldehydes and Aldehydes are generally. Ketones more reactive than ketones in nucleophilic addition reactions • The transition state for addition is less crowded and lower in energy for an aldehyde (a) than for a ketone (b) • Aldehydes have one large substituent bonded to the C=O: ketones have two
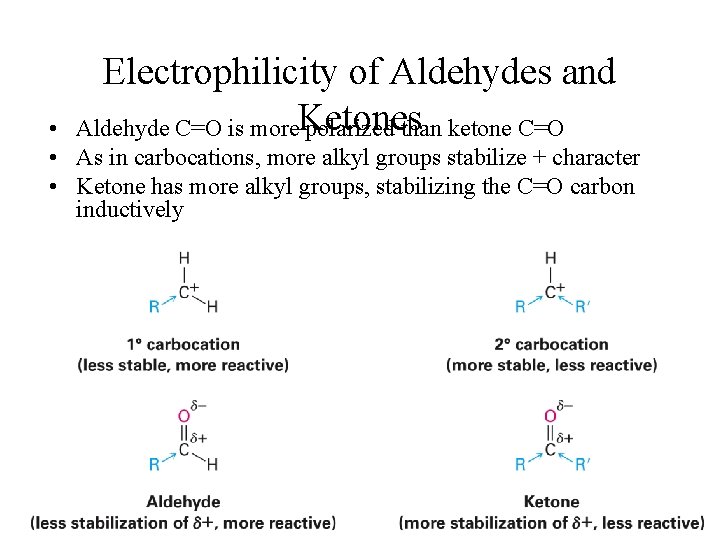
Electrophilicity of Aldehydes and Aldehyde C=O is more. Ketones polarized than ketone C=O • • As in carbocations, more alkyl groups stabilize + character • Ketone has more alkyl groups, stabilizing the C=O carbon inductively
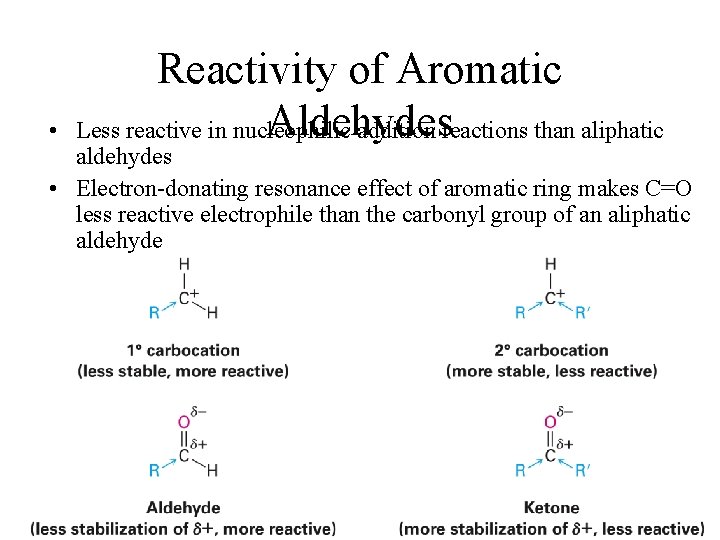
• Reactivity of Aromatic Aldehydes Less reactive in nucleophilic addition reactions than aliphatic aldehydes • Electron-donating resonance effect of aromatic ring makes C=O less reactive electrophile than the carbonyl group of an aliphatic aldehyde

Dangers of HCN Hydrogen cyanide (HCN) is highly volatile liquid (boiling point 26 °C), which has a faint bitter almond smell. In solution, hydrogen cyanide partially dissociates: HCN(aq) H+(aq) + CN-(aq) Hydrogen cyanide is highly toxic because it inhibits a mitochondrial enzyme that is essential for respiration. Being so volatile and flammable, it is difficult to handle safely. A safer alternative is KCN, which is a solid at room temperature and is therefore easier to handle. An acidified solution contains both the H+ and CN- ions.
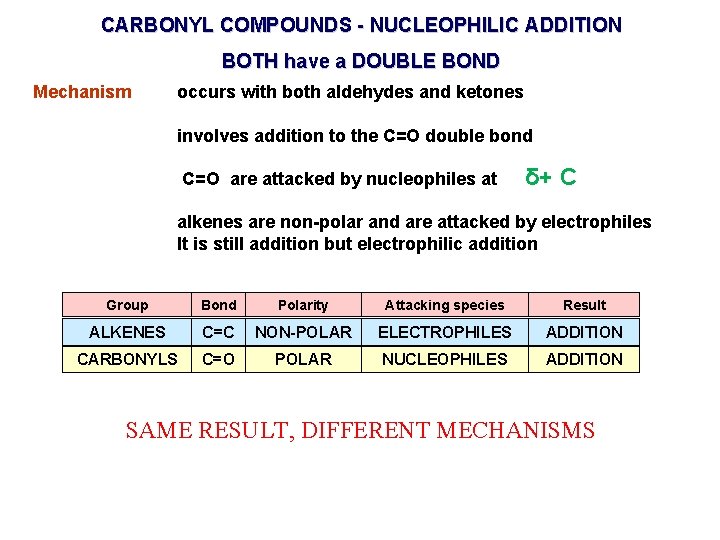
CARBONYL COMPOUNDS - NUCLEOPHILIC ADDITION BOTH have a DOUBLE BOND Mechanism occurs with both aldehydes and ketones involves addition to the C=O double bond C=O are attacked by nucleophiles at δ+ C alkenes are non-polar and are attacked by electrophiles It is still addition but electrophilic addition Group Bond Polarity Attacking species Result ALKENES C=C NON-POLAR ELECTROPHILES ADDITION CARBONYLS C=O POLAR NUCLEOPHILES ADDITION SAME RESULT, DIFFERENT MECHANISMS

CARBONYL COMPOUNDS - NUCLEOPHILIC ADDITION CH 3 CHO + HCN ——> CH 3 CH(OH)CN 2 -hydroxypropanenitrile STEP 1 Step 1: CN¯ acts as a nucleophile, & attacks δ breaks; a pair of electrons goes onto the O STEP 2 + Carbon. One of the C=O bonds Step 2: A pair of electrons is used to form a bond with H+. Overall, there has been addition of HCN
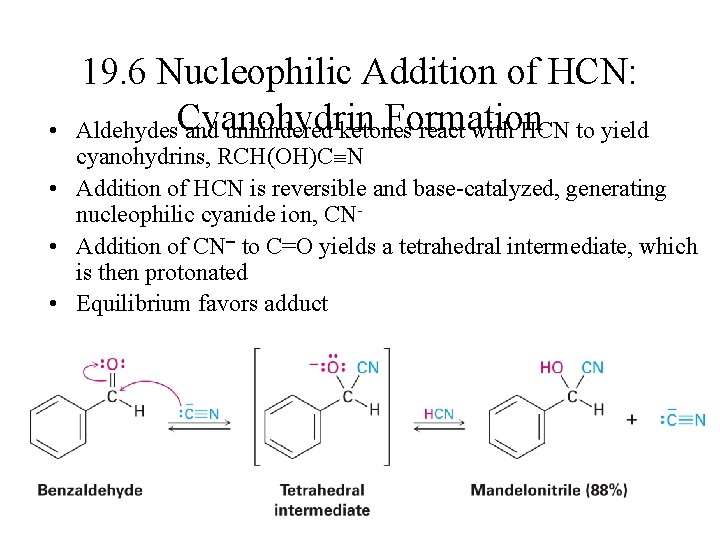
• 19. 6 Nucleophilic Addition of HCN: Formation Aldehydes. Cyanohydrin and unhindered ketones react with HCN to yield cyanohydrins, RCH(OH)C N • Addition of HCN is reversible and base-catalyzed, generating nucleophilic cyanide ion, CN • Addition of CN to C=O yields a tetrahedral intermediate, which is then protonated • Equilibrium favors adduct
CARBONYL COMPOUNDS - NUCLEOPHILIC ADDITION ANIMATED MECHANISM TO SHOW DIFFERENT ISOMERS ARE FORMED

Electrophilic H 2 Addition using Pt catalyst

Reduction Reactions Aldehydes 1 o Alcohol Ketone 2 o Alcohol
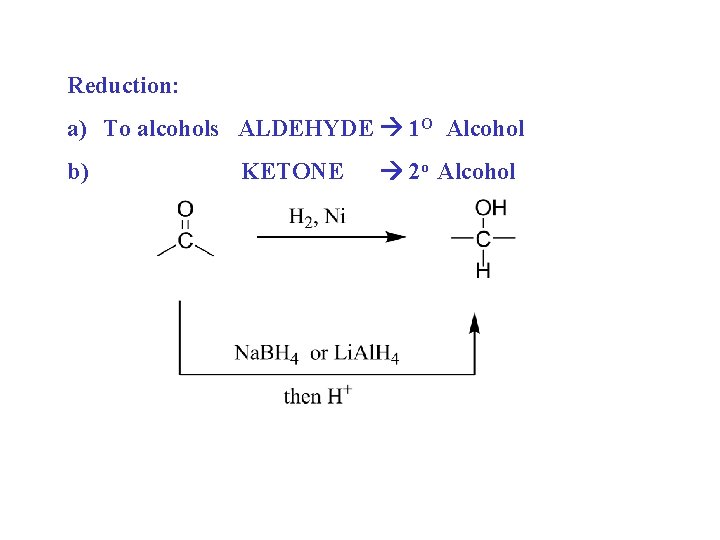
Reduction: a) To alcohols ALDEHYDE 1 O Alcohol b) KETONE 2 o Alcohol
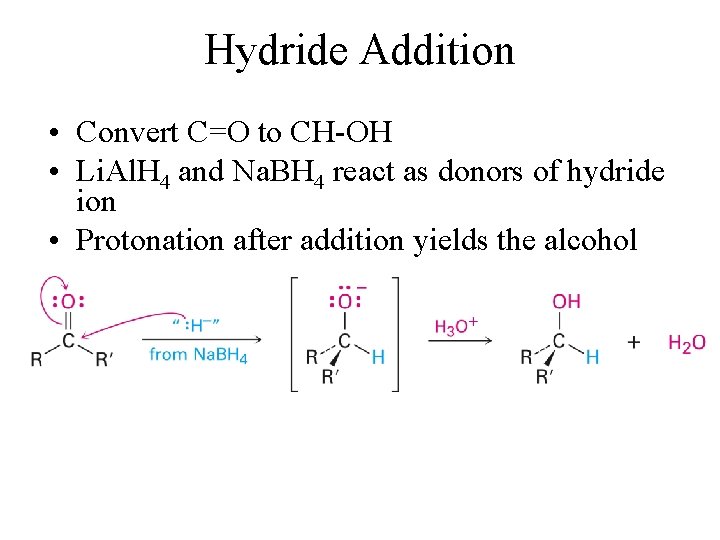
Hydride Addition • Convert C=O to CH-OH • Li. Al. H 4 and Na. BH 4 react as donors of hydride ion • Protonation after addition yields the alcohol
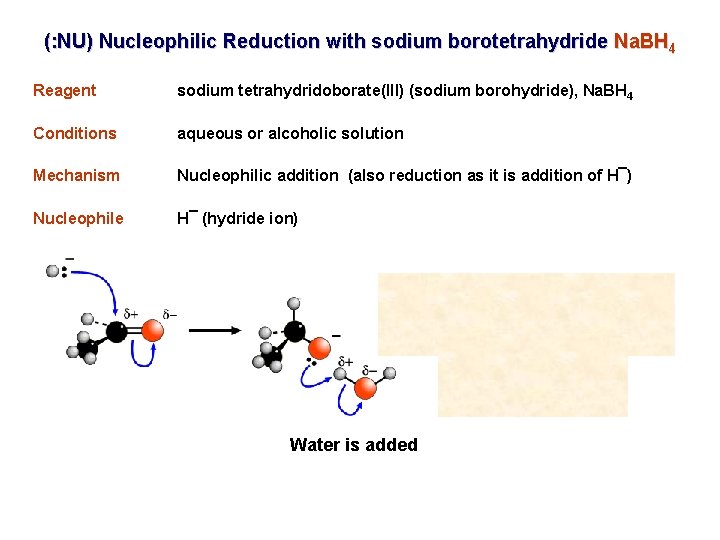
(: NU) Nucleophilic Reduction with sodium borotetrahydride Na. BH 4 Reagent sodium tetrahydridoborate(III) (sodium borohydride), Na. BH 4 Conditions aqueous or alcoholic solution Mechanism Nucleophilic addition (also reduction as it is addition of H¯) Nucleophile H¯ (hydride ion) Water is added
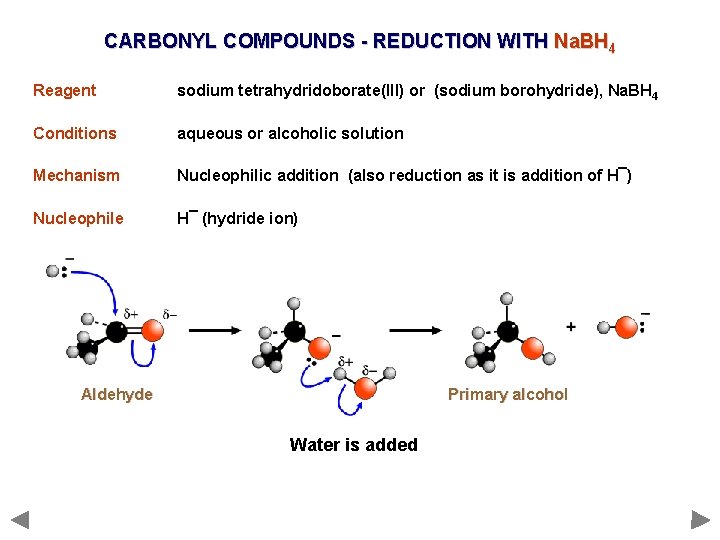
CARBONYL COMPOUNDS - REDUCTION WITH Na. BH 4 Reagent sodium tetrahydridoborate(III) or (sodium borohydride), Na. BH 4 Conditions aqueous or alcoholic solution Mechanism Nucleophilic addition (also reduction as it is addition of H¯) Nucleophile H¯ (hydride ion) Aldehyde Primary alcohol Water is added
CARBONYL COMPOUNDS - REDUCTION WITH Na. BH 4 ANIMATED MECHANISM

CARBONYL COMPOUNDS - REDUCTION WITH HYDROGEN Reagent hydrogen Conditions catalyst - nickel or platinum Reaction type Hydrogenation, reduction Product(s) Alcohols Aldehydes are REDUCED to primary (1°) alcohols. Ketones are REDUCED to secondary (2°) alcohols. Equation(s) CH 3 CHO + H 2 CH 3 COCH 3 + H 2 Note ——> CH 3 CH 2 OH ——> CH 3 CHOHCH 3 Hydrogen also reduces C=C bonds but via Electrophilic Addition CH 2 = CHCHO + 2 H 2 ——> CH 3 CH 2 OH


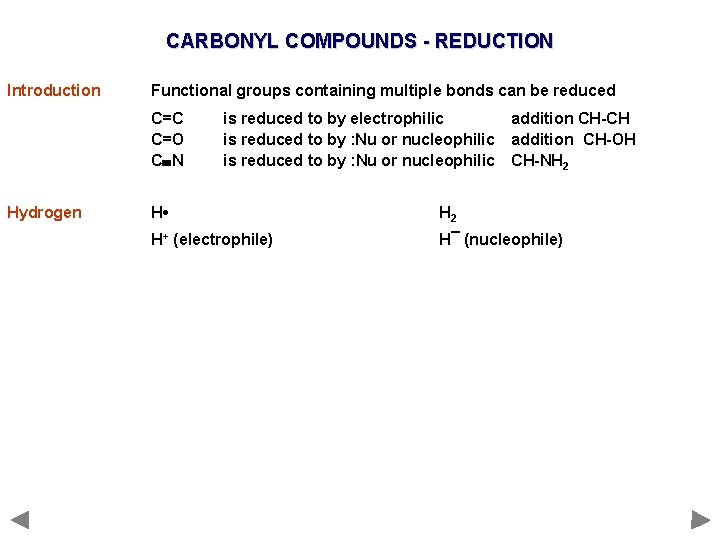
CARBONYL COMPOUNDS - REDUCTION Introduction Functional groups containing multiple bonds can be reduced C=C C=O C N Hydrogen is reduced to by electrophilic is reduced to by : Nu or nucleophilic addition CH-CH addition CH-OH CH-NH 2 H • H 2 H+ (electrophile) H¯ (nucleophile)
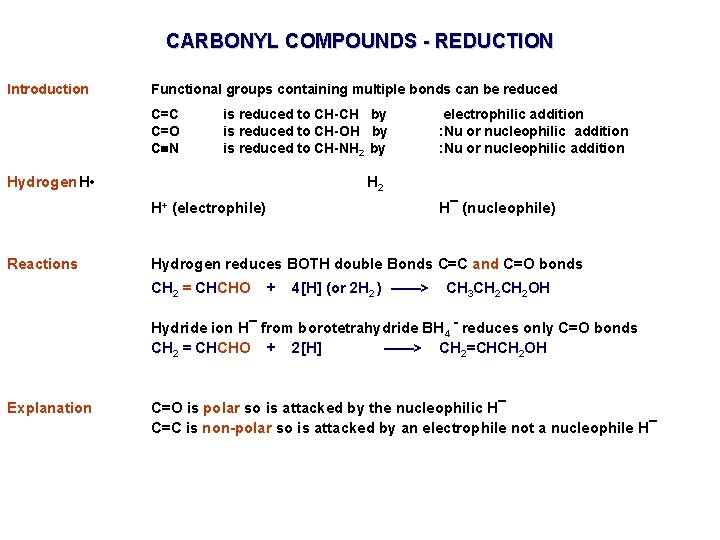
CARBONYL COMPOUNDS - REDUCTION Introduction Functional groups containing multiple bonds can be reduced C=C C=O C N is reduced to CH-CH by is reduced to CH-OH by is reduced to CH-NH 2 by Hydrogen H • H 2 H+ (electrophile) Reactions electrophilic addition : Nu or nucleophilic addition H¯ (nucleophile) Hydrogen reduces BOTH double Bonds C=C and C=O bonds CH 2 = CHCHO + 4[H] (or 2 H 2 ) ——> CH 3 CH 2 OH Hydride ion H¯ from borotetrahydride BH 4 - reduces only C=O bonds CH 2 = CHCHO + 2[H] ——> CH 2=CHCH 2 OH Explanation C=O is polar so is attacked by the nucleophilic H¯ C=C is non-polar so is attacked by an electrophile not a nucleophile H¯
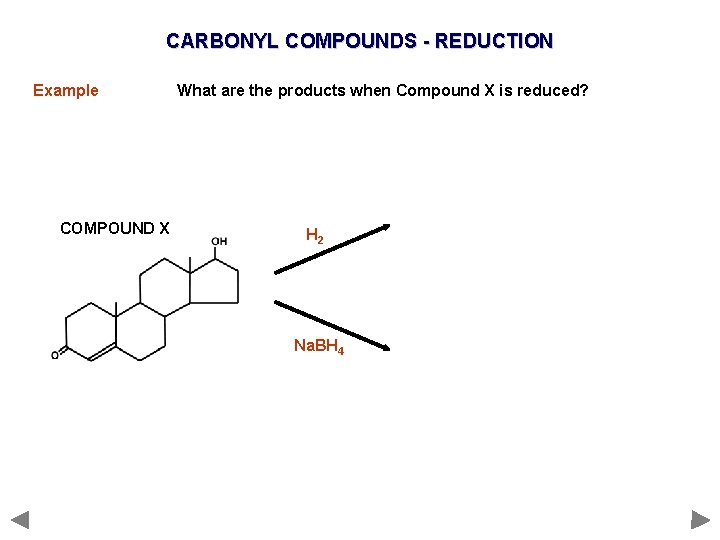
CARBONYL COMPOUNDS - REDUCTION Example COMPOUND X What are the products when Compound X is reduced? H 2 Na. BH 4

CARBONYL COMPOUNDS - REDUCTION Example What are the products when Compound X is reduced? COMPOUND X H 2 Na. BH 4 C=O is polar so is attacked by the nucleophilic H¯ C=C is non-polar so is not attacked by the nucleophilic H¯

2, 4 -DINITROPHENYLHYDRAZINE TEST for C=O (N 2 H 4 is hydrazine) Structure C 6 H 3(NO 2)2 NHNH 2 Use reacts with carbonyl compounds (aldehydes and ketones) used as a simple test for aldehydes and ketones makes orange crystalline solid, if the colour appears we have a C=O Identification / characterisation A simple way of characterising a compound (finding out what it is)
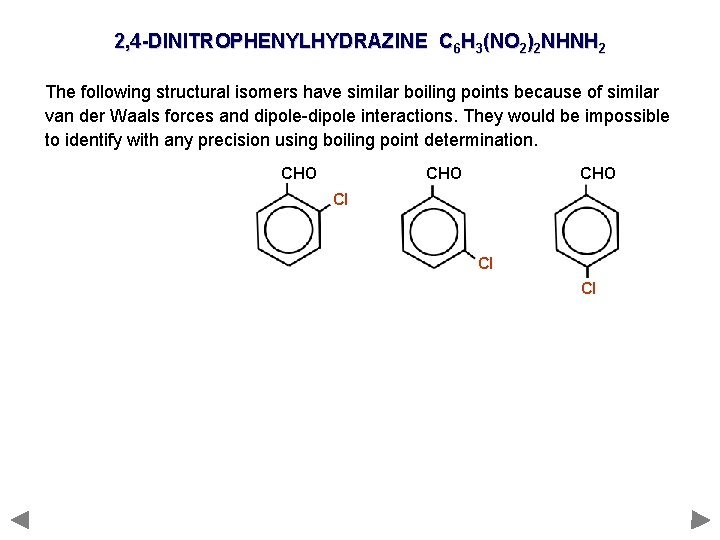
2, 4 -DINITROPHENYLHYDRAZINE C 6 H 3(NO 2)2 NHNH 2 The following structural isomers have similar boiling points because of similar van der Waals forces and dipole-dipole interactions. They would be impossible to identify with any precision using boiling point determination. CHO CHO Cl Cl Cl
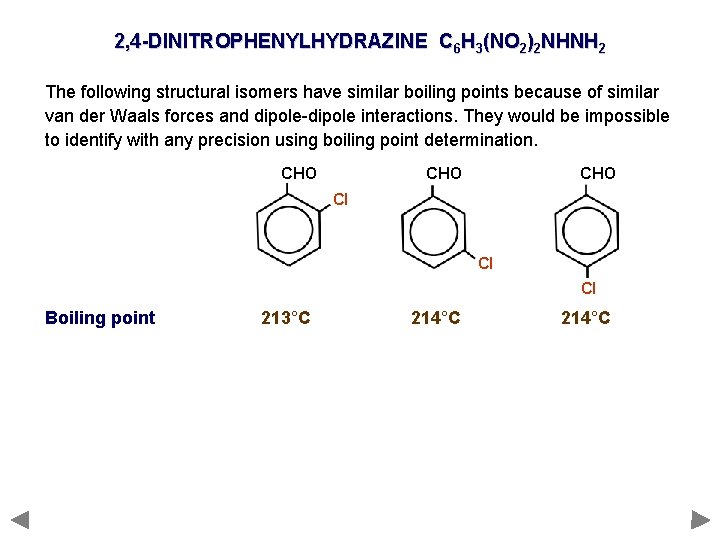
2, 4 -DINITROPHENYLHYDRAZINE C 6 H 3(NO 2)2 NHNH 2 The following structural isomers have similar boiling points because of similar van der Waals forces and dipole-dipole interactions. They would be impossible to identify with any precision using boiling point determination. CHO CHO Cl Cl Cl Boiling point 213°C 214°C
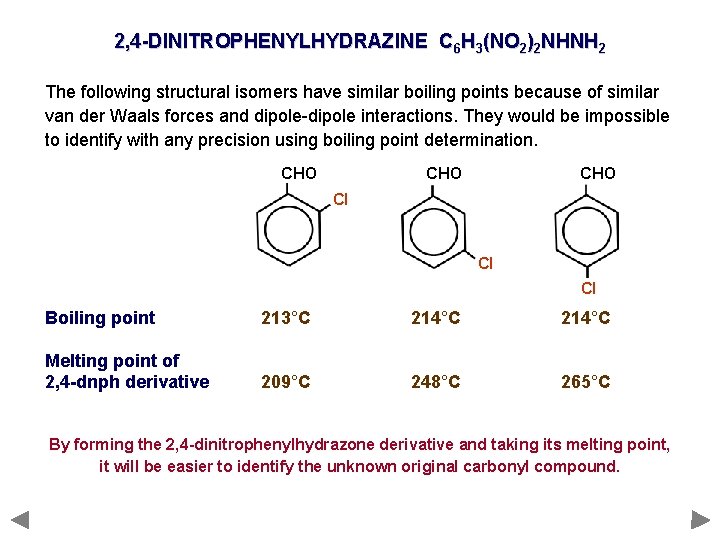
2, 4 -DINITROPHENYLHYDRAZINE C 6 H 3(NO 2)2 NHNH 2 The following structural isomers have similar boiling points because of similar van der Waals forces and dipole-dipole interactions. They would be impossible to identify with any precision using boiling point determination. CHO CHO Cl Cl Cl Boiling point 213°C 214°C Melting point of 2, 4 -dnph derivative 209°C 248°C 265°C By forming the 2, 4 -dinitrophenylhydrazone derivative and taking its melting point, it will be easier to identify the unknown original carbonyl compound.

2, 4 -DINITROPHENYLHYDRAZINE C 6 H 3(NO 2)2 NHNH 2 The following structural isomers have similar boiling points because of similar van der Waals forces and dipole-dipole interactions. They would be impossible to identify with any precision using boiling point determination. CHO CHO Cl Cl Cl Boiling point (alone) 213°C 214°C Melting point of 2, 4 -dnph with C=O 209°C 248°C 265°C
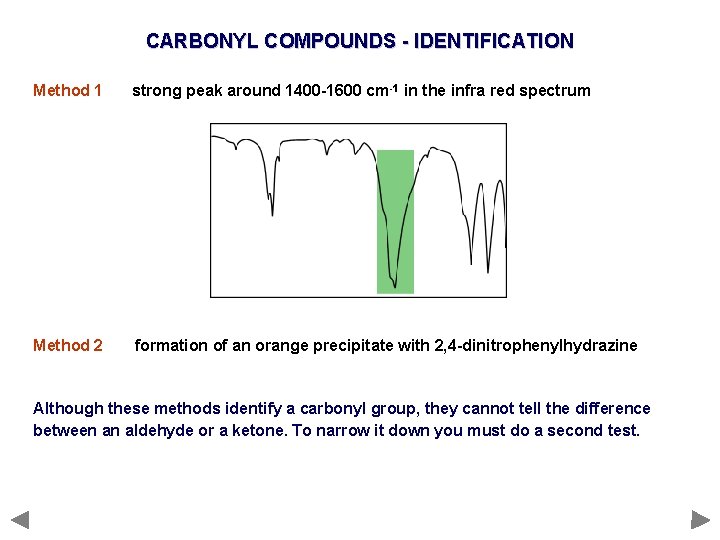
CARBONYL COMPOUNDS - IDENTIFICATION Method 1 strong peak around 1400 -1600 cm-1 in the infra red spectrum Method 2 formation of an orange precipitate with 2, 4 -dinitrophenylhydrazine Although these methods identify a carbonyl group, they cannot tell the difference between an aldehyde or a ketone. To narrow it down you must do a second test.

Spectroscopy of Aldehydes and Ketones • Infrared Spectroscopy • Aldehydes and ketones show a strong C=O peak 1660 to 1770 cm 1
CARBONYL COMPOUNDS - IDENTIFICATION Differentiation Tollen’s Reagent Fehling’s Solution to distinguish aldehydes from ketones, use a mild oxidising agent ammoniacal silver nitrate mild oxidising agent which will oxidise aldehydes but not ketones contains the diammine silver(I) ion - [Ag(NH 3)2 ]+ the silver(I) ion is reduced to silver Ag+(aq) + e¯ ——> Ag(s) the test is known as THE SILVER MIRROR TEST contains a copper(II) complex ion giving a blue solution on warming, it will oxidise aliphatic (but not aromatic) aldehydes the copper(II) is reduced to copper(I) a red precipitate of copper(I) oxide, Cu 2 O, is formed The silver mirror test is the better alternative as it works with all aldehydes Ketones do not react with Tollen’s Reagent or Fehling’s Solution
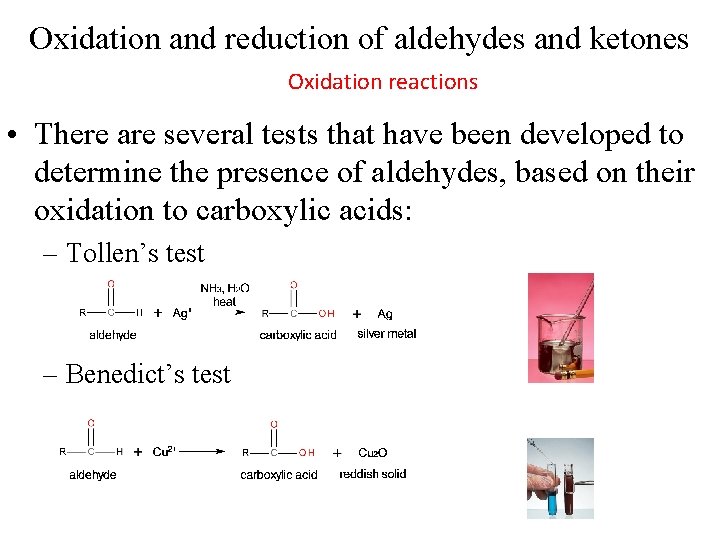
Oxidation and reduction of aldehydes and ketones Oxidation reactions • There are several tests that have been developed to determine the presence of aldehydes, based on their oxidation to carboxylic acids: – Tollen’s test – Benedict’s test
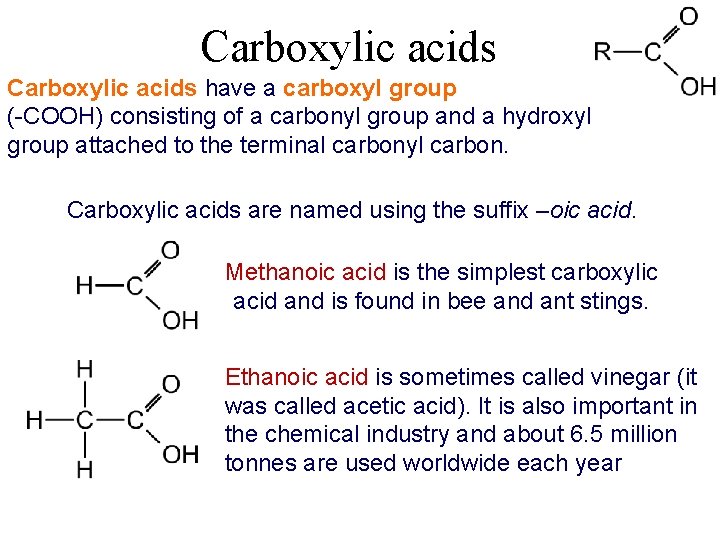
Carboxylic acids have a carboxyl group (-COOH) consisting of a carbonyl group and a hydroxyl group attached to the terminal carbonyl carbon. Carboxylic acids are named using the suffix –oic acid. Methanoic acid is the simplest carboxylic acid and is found in bee and ant stings. Ethanoic acid is sometimes called vinegar (it was called acetic acid). It is also important in the chemical industry and about 6. 5 million tonnes are used worldwide each year

Forming the carboxylate ion Carboxylic acids are weak acids and partially dissociate in aqueous solution: carboxylate ion The negative charge is delocalized across the carboxylate group, resulting in a more stable ion. The delocalization is represented by adding a second dotted line to the carbon oxygen bonds, which are both equivalent.

Reactivity of carboxylic acids δ- The reactivity of carboxylic acids results, in part, from the polarization of its bonds. δ+ δ- δ+ The 3 types of reactions of carboxylic acids (C. A. ) include: l neutralisation – the C. A. loses a H+ to form a carboxylate ion (-COO - ) Ex: CH 3 COOH + OH- H 2 O + CH 3 COO - l nucleophilic substitution – the positively-charged carbon is attacked by a nucleophile, resulting in substitution of the OH group l esterification – reaction with an alcohol to form an ester.
Reactions with carbonates Carboxylic acids are the only organic compounds that are strong enough acids to react with either sodium carbonate or sodium hydrogencarbonate. Reaction of ethanoic acid and sodium carbonate: 2 CH 3 COOH + Na 2 CO 3 → 2 CH 3 COONa + CO 2 + H 2 O sodium ethanoate Reaction of methanoic acid and sodium hydrogencarbonate: HCOOH + Na. HCO 3 → HCOONa + CO 2 + H 2 O sodium methanoate The formation of bubbles of carbon dioxide gas makes these reactions useful as a test for carboxylic acids.

Reactions with alkalis Carboxylic acids will react with alkalis, such as sodium hydroxide, in a neutralization reaction: RCOOH + Na. OH → RCOONa + H 2 O Reaction of propanoic acid and sodium hydroxide: CH 3 CH 2 COOH + Na. OH → CH 3 CH 2 COONa + H 2 O sodium propanoate Reaction of butanoic acid and potassium hydroxide: CH 3 CH 2 COOH + KOH → CH 3 CH 2 COOK + H 2 O potassium butanoate

Carboxylic acids reaction with Alcohol C. A. + R-OH R-CO 2 -R (ester) + H 2 O Esters contain this group of atoms: carboxylic acid + alcohol ester + water propanoic acid + methanol methyl propanoate + water CH 3 CH 2 COOH + + H 2 O + CH 3 OH CH 3 CH 2 COOCH 3 +
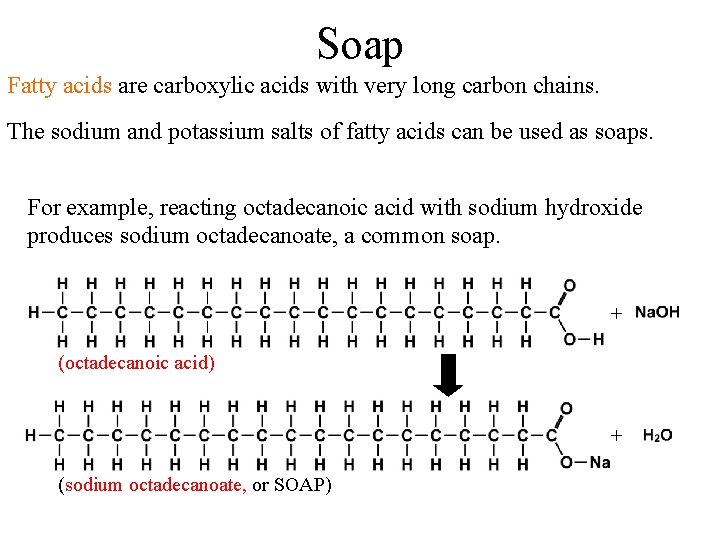
Soap Fatty acids are carboxylic acids with very long carbon chains. The sodium and potassium salts of fatty acids can be used as soaps. For example, reacting octadecanoic acid with sodium hydroxide produces sodium octadecanoate, a common soap. + (octadecanoic acid) + (sodium octadecanoate, or SOAP)

What are esters? The Big Fat! Esters have characteristic smells, and are often used in flavourings and perfumes. For example: l propyl ethanoate smells of pears l butyl butanoate smells of pineapple l methyl butanoate smells of apple.
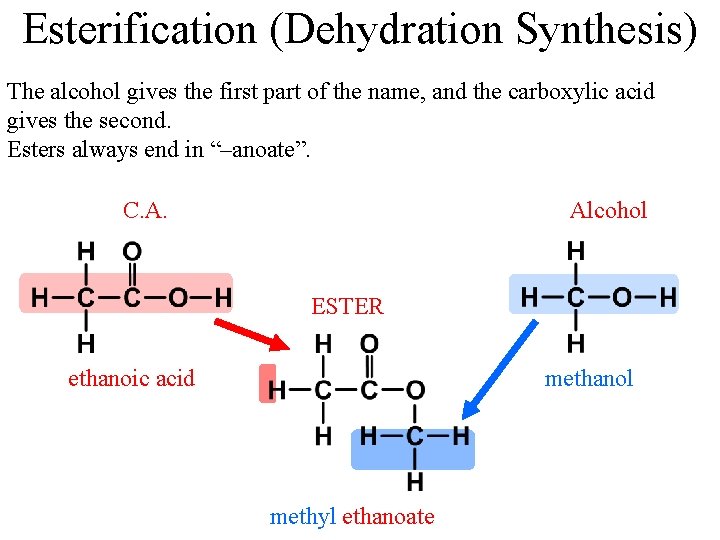
Esterification (Dehydration Synthesis) The alcohol gives the first part of the name, and the carboxylic acid gives the second. Esters always end in “–anoate”. C. A. Alcohol ESTER ethanoic acid methanol methyl ethanoate
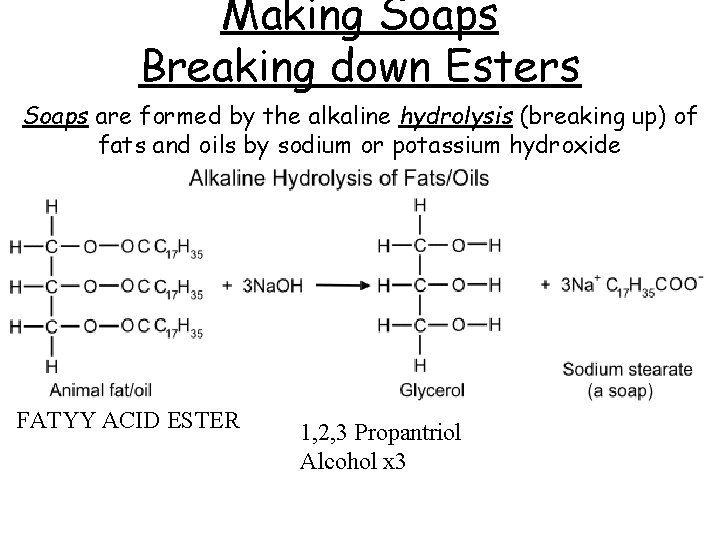
Making Soaps Breaking down Esters Soaps are formed by the alkaline hydrolysis (breaking up) of fats and oils by sodium or potassium hydroxide FATYY ACID ESTER 1, 2, 3 Propantriol Alcohol x 3
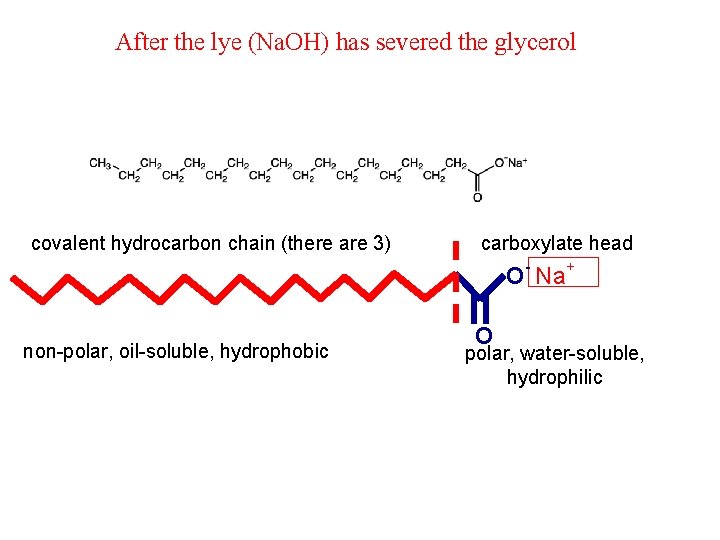
After the lye (Na. OH) has severed the glycerol covalent hydrocarbon chain (there are 3) carboxylate head O- Na+ non-polar, oil-soluble, hydrophobic O polar, water-soluble, hydrophilic
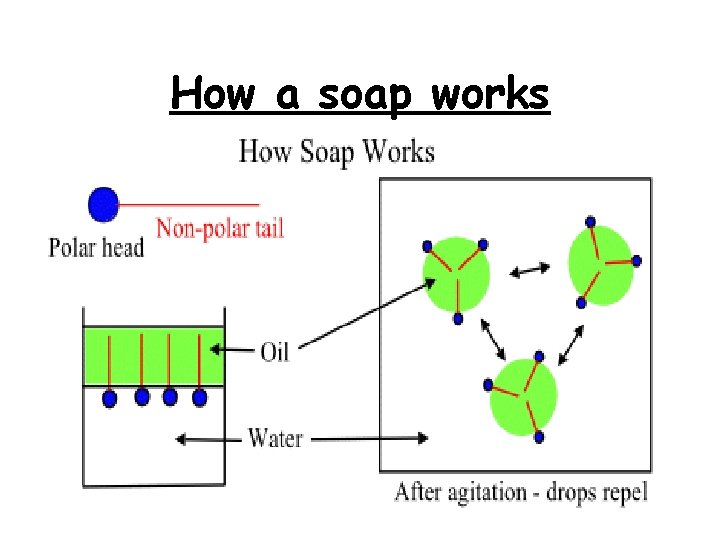
How a soap works

Mechanism of stain/dirt removal Roll-up mechanism The hydrophobic tails ‘burrow’ into the droplet of oil or grease. The hydrophilic heads are left to face the surrounding water. This results in the formation of a ball-like structure (a micelle). The non-polar substances, such as oil or grease, are held inside the ball and suspended in water, to be washed away.

Soapless detergents When soap is used in hard water, a white solid precipitate we call scum forms. This is because charged calcium and magnesium ions present in the hard water react with soap to form an insoluble substance. Detergent molecules simply replace the head of the soap -COO - carboxylate group to -SO 3 - called sulphonate, it is now detergent, not soap. The calcium ions will not ppt with the -SO 3 -

Emulsions Soap for food • Ok, yuck, not a soap like in the shower soap, but it has the same ACTION • An emulsion contains small droplets of one liquid dispersed in another liquid. • Emulsions in Food – Emulsions in food are mixtures of oil and water. To prevent oil and water components separating into layers, a soap-like molecule known as an emulsifier is added.

Biodiesel • Biofuel: Fuel made from biological ingredients instead of fossil fuels – Starting ingredients range from corn to soybeans to animal fat – Non-toxic and renewable Soybean Canola Peanut Why? These compounds contain triacylglycerols, or fat

Abbreviated process • Transesterification – Fat is reacted with an alcohol, usually methanol. So the glycerol is swapped with the alcohol. – Alcohol for Alcohol – Sodium or potassium hydroxide is used as a catalyst

Pros • • • Can help reduce dependence on oil Helps lubricate the engine, reducing wear Can be used in virtually any diesel operating vehicle biodegradable, renewable Non-toxic
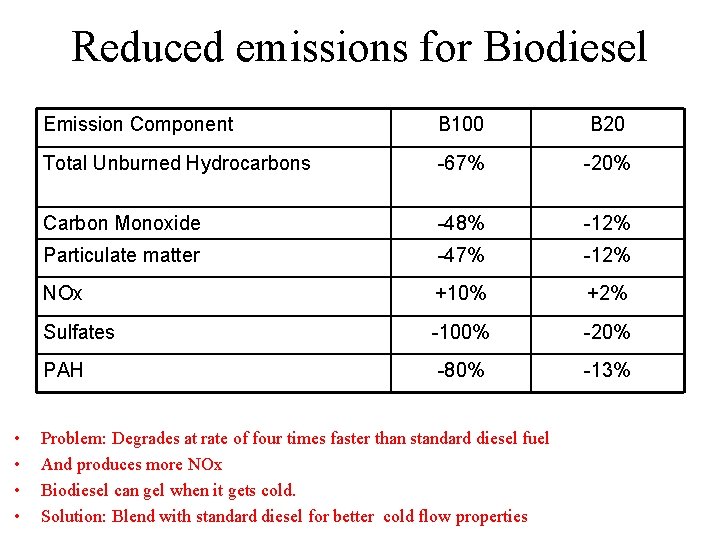
Reduced emissions for Biodiesel • • Emission Component B 100 B 20 Total Unburned Hydrocarbons -67% -20% Carbon Monoxide -48% -12% Particulate matter -47% -12% NOx +10% +2% Sulfates -100% -20% PAH -80% -13% Problem: Degrades at rate of four times faster than standard diesel fuel And produces more NOx Biodiesel can gel when it gets cold. Solution: Blend with standard diesel for better cold flow properties

Algae? • How? – Grow colonies in presence of sunlight – Starve them of nitrogen – Filter out cell membranes and organelles – Solvent to separate out the fats – Purify fats, evaporate solvent • Aquaflow in New Zealand • Cold pressing: how oil is retrieved from plants • 140 billion gallons of biodiesel every year – Soybeans: 3 billion acres – Canola: 1 billion acres – Algae: 95 million acres – only would need about size of Maryland

Glycerin By-Product Uses • Soaps • Nitration: produces nitroglycerin • Heart disease drug: 0. 6 mg • Cosmetics

Concerns • Operate in cold weather? – Biodiesel can gel when it gets cold • Solution – Blend with standard diesel for better cold flow properties
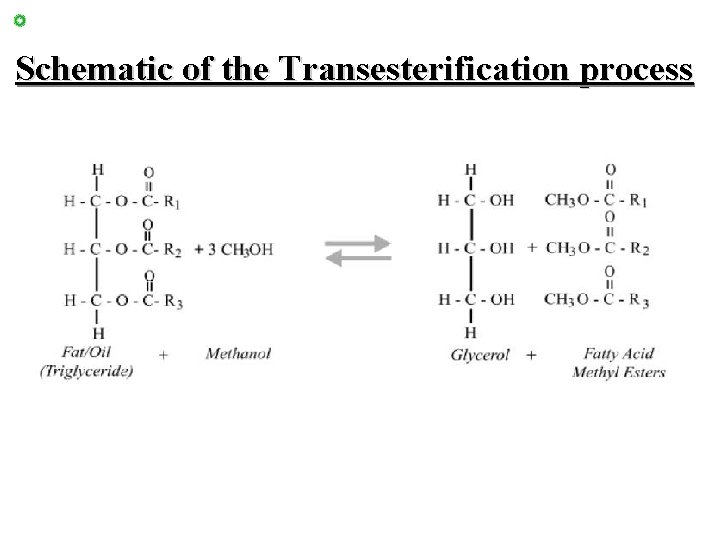
Schematic of the Transesterification process
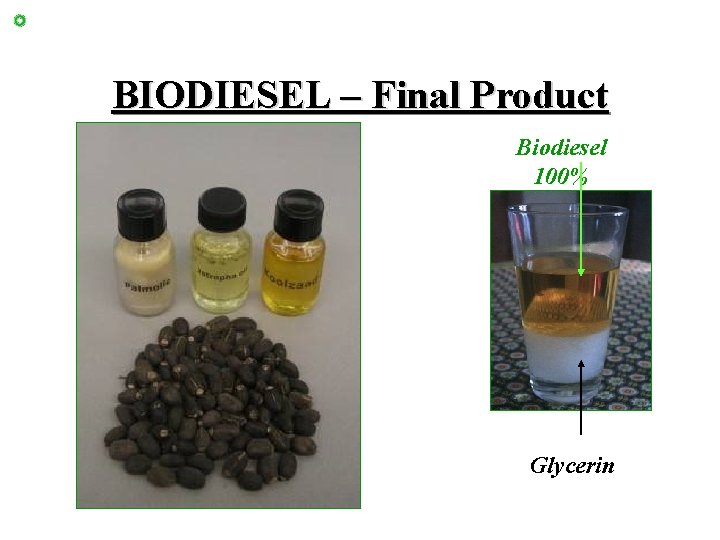
BIODIESEL – Final Product Biodiesel 100% Glycerin
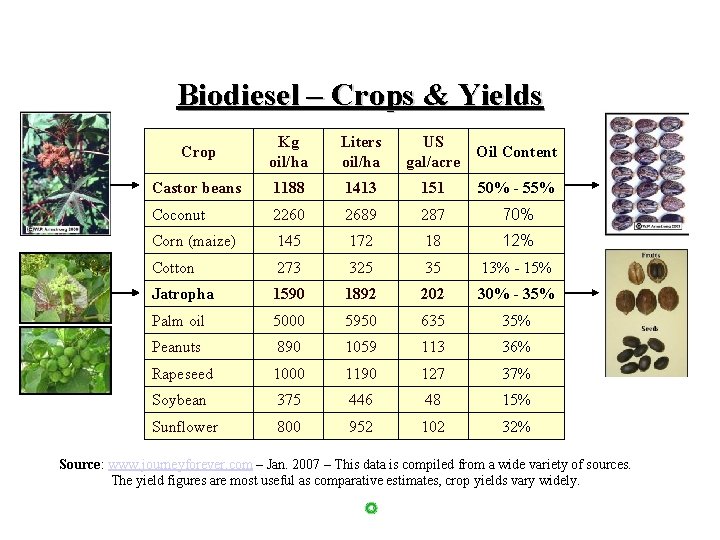
Biodiesel – Crops & Yields Crop Kg oil/ha Liters oil/ha US gal/acre Oil Content Castor beans 1188 1413 151 50% - 55% Coconut 2260 2689 287 70% Corn (maize) 145 172 18 12% Cotton 273 325 35 13% - 15% Jatropha 1590 1892 202 30% - 35% Palm oil 5000 5950 635 35% Peanuts 890 1059 113 36% Rapeseed 1000 1190 127 37% Soybean 375 446 48 15% Sunflower 800 952 102 32% Source: www. journeyforever. com – Jan. 2007 – This data is compiled from a wide variety of sources. The yield figures are most useful as comparative estimates, crop yields vary widely.

This…. …or this?

Using US maize to produce ethanol increased tortilla price in Mexico
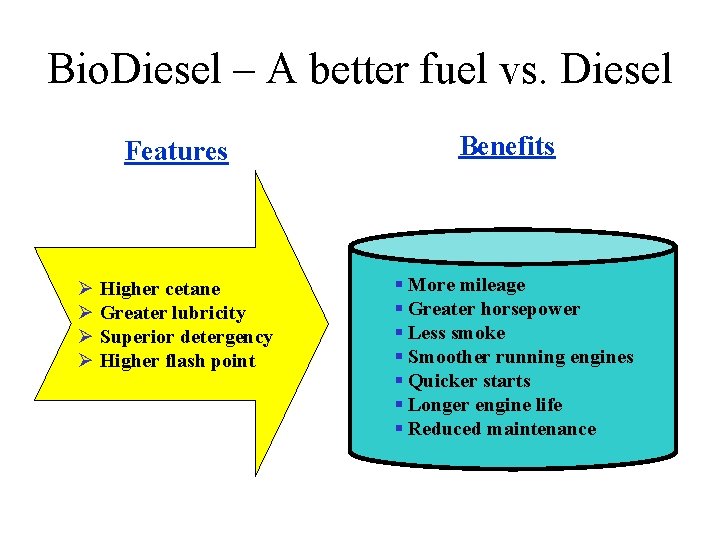
Bio. Diesel – A better fuel vs. Diesel Features Ø Higher cetane Ø Greater lubricity Ø Superior detergency Ø Higher flash point Benefits § More mileage § Greater horsepower § Less smoke § Smoother running engines § Quicker starts § Longer engine life § Reduced maintenance
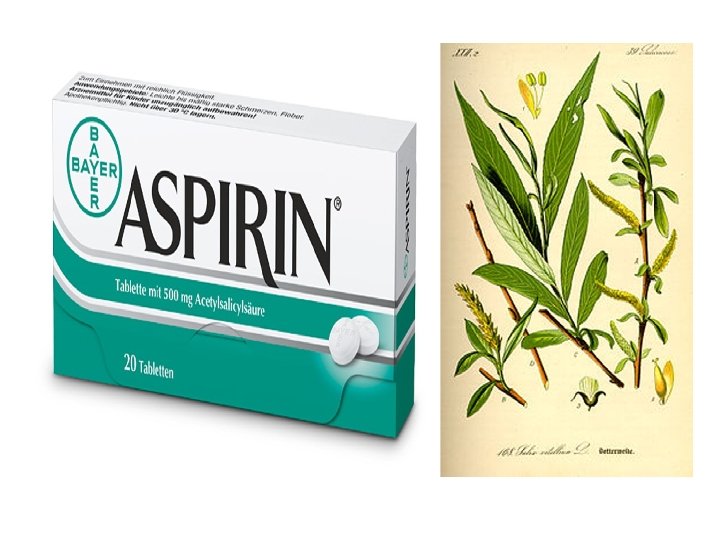

History • • Salicin, derived from Willow Bark, had been used since the time of the ancient Greek philosopher, Hippocrates, to relieve dull aches and pains. Salicin is metabolized by the body to produce salicylic acid, which has similar anti-inflammatory properties to that of aspirin, but causes extreme stomach discomfort, even stomach bleeding. In 1897, Felix Hoffman, a researcher from Bayer AG synthesized acetylsalicylic acid from salicin derived from meadowsweet. In 1919, Bayer AG lost Aspirin as a registered trademark in the US, the UK, France, and Russia due to war reparations imposed by the Allies.
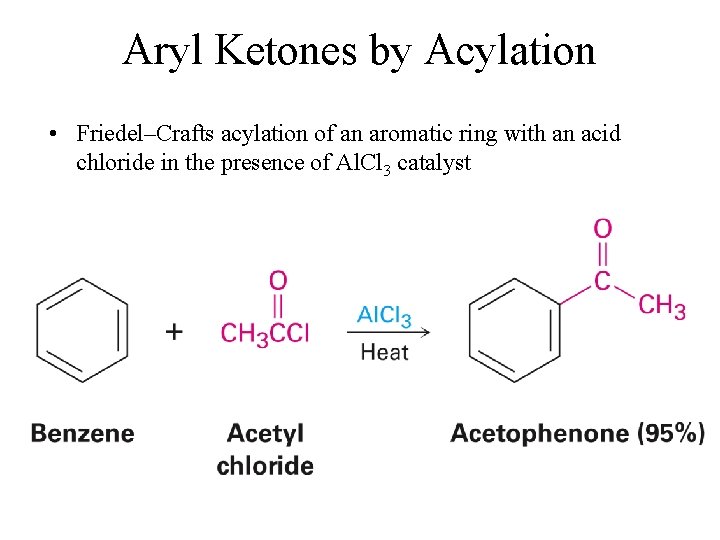
Aryl Ketones by Acylation • Friedel–Crafts acylation of an aromatic ring with an acid chloride in the presence of Al. Cl 3 catalyst
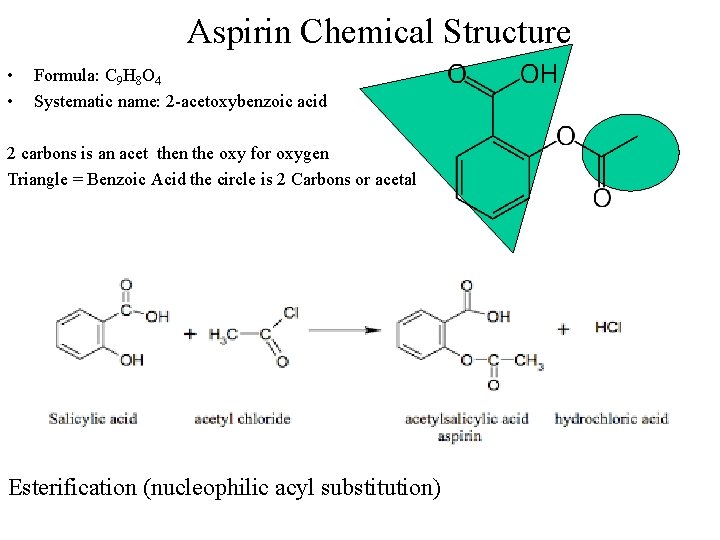
Aspirin Chemical Structure • • Formula: C 9 H 8 O 4 Systematic name: 2 -acetoxybenzoic acid 2 carbons is an acet then the oxy for oxygen Triangle = Benzoic Acid the circle is 2 Carbons or acetal Esterification (nucleophilic acyl substitution)
- Slides: 89