Aldehydes and Ketones Chapter 5 Aldehydes and Ketones











- Slides: 36

Aldehydes and Ketones Chapter 5

Aldehydes and Ketones l Functional group is O The carbonyl ll group C Aldehyde = Carbonyl group is 1° Ketone = Carbonyl group is 2°

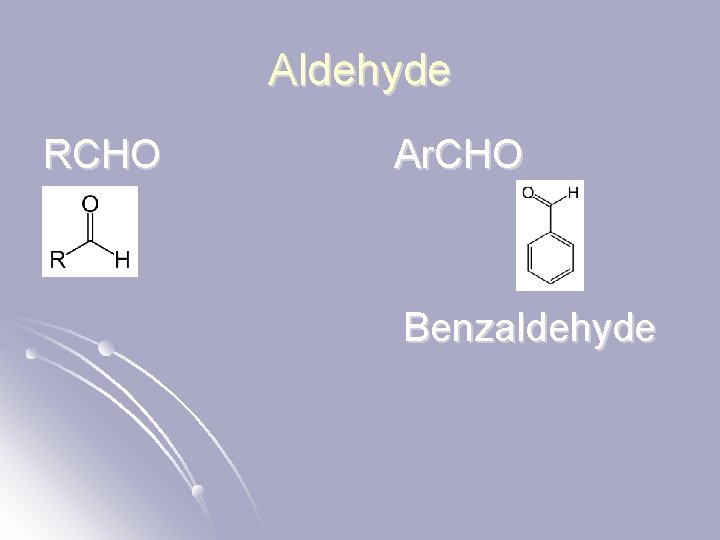
Aldehyde RCHO Ar. CHO Benzaldehyde

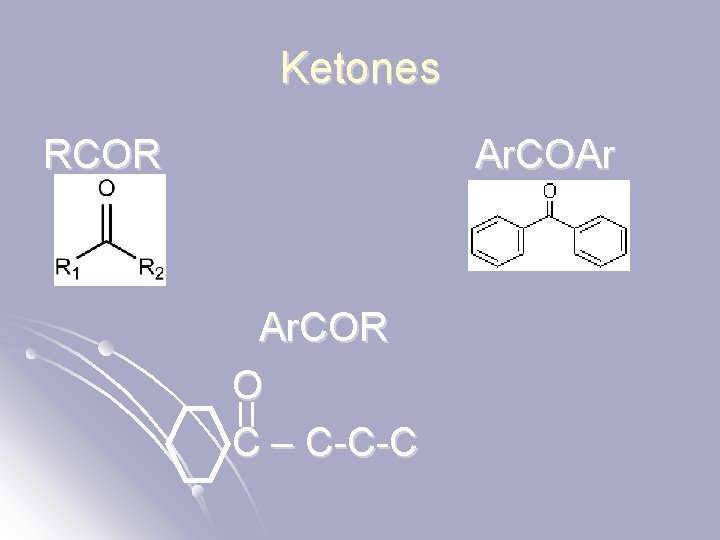
Ketones RCOR Ar. COAr Ar. COR O C – C-C-C

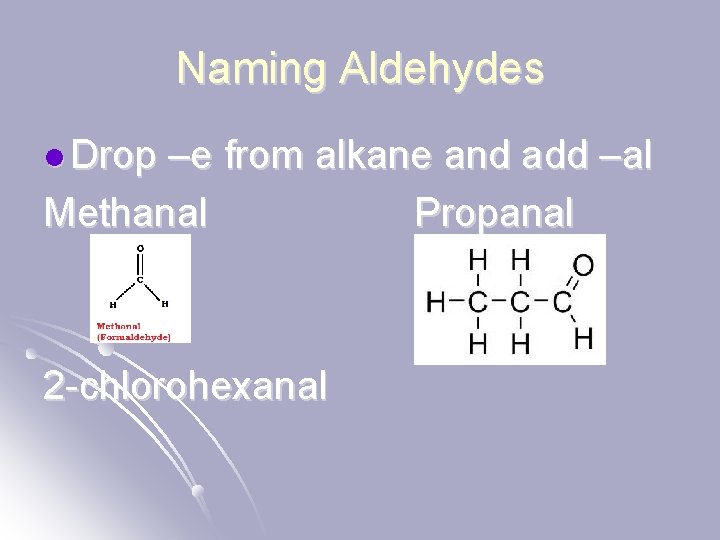
Naming Aldehydes l Drop –e from alkane and add –al Methanal Propanal 2 -chlorohexanal

Practice

Naming Ketones (2 ways) 1. 2. IUPAC - # chain from the end closest to the carbonyl, give the carbon #, drop –e add –one Name the groups on either side of the carbonyl and follow with ketone list groups by order of sixe of molar mass (smaller 1 st)

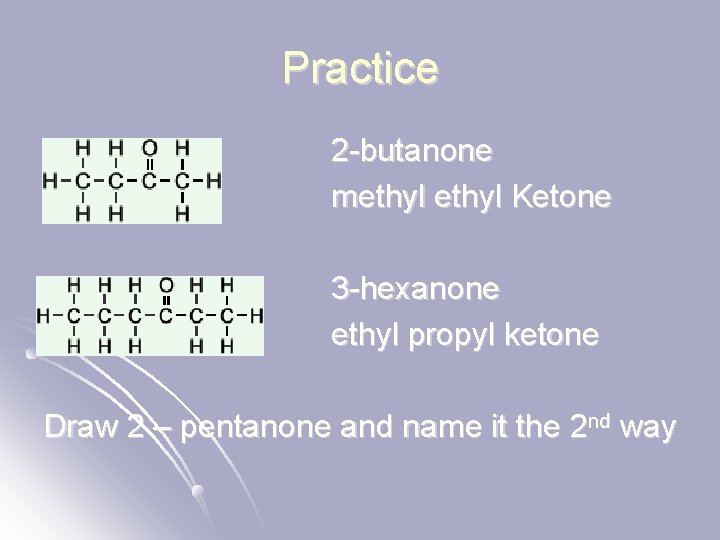
Practice 2 -butanone methyl Ketone 3 -hexanone ethyl propyl ketone Draw 2 – pentanone and name it the 2 nd way

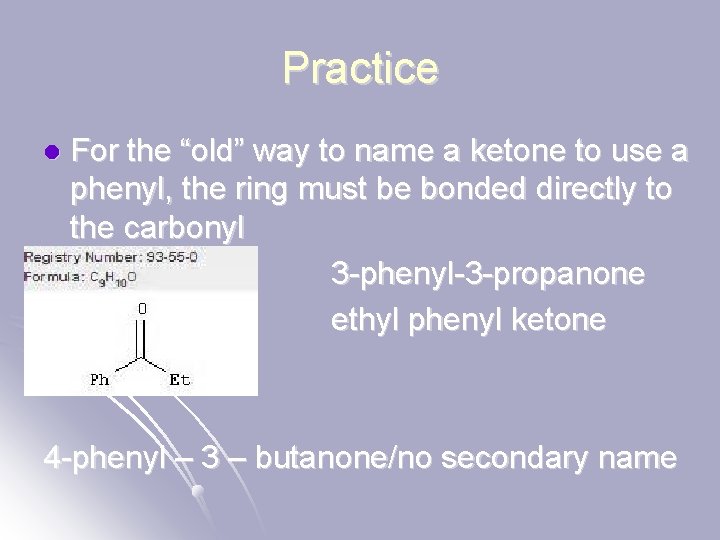
Practice l For the “old” way to name a ketone to use a phenyl, the ring must be bonded directly to the carbonyl 3 -phenyl-3 -propanone ethyl phenyl ketone 4 -phenyl – 3 – butanone/no secondary name

Practice

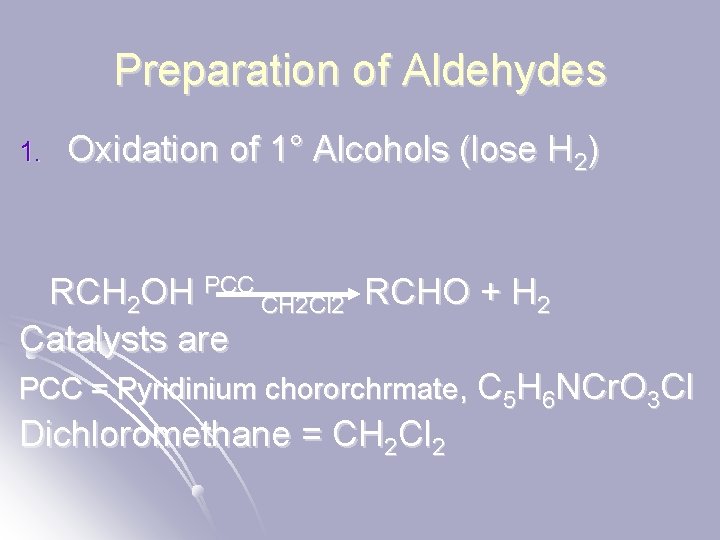
Preparation of Aldehydes 1. Oxidation of 1° Alcohols (lose H 2) RCH 2 OH PCC CH 2 Cl 2 RCHO + H 2 Catalysts are PCC = Pyridinium chororchrmate, C 5 H 6 NCr. O 3 Cl Dichloromethane = CH 2 Cl 2

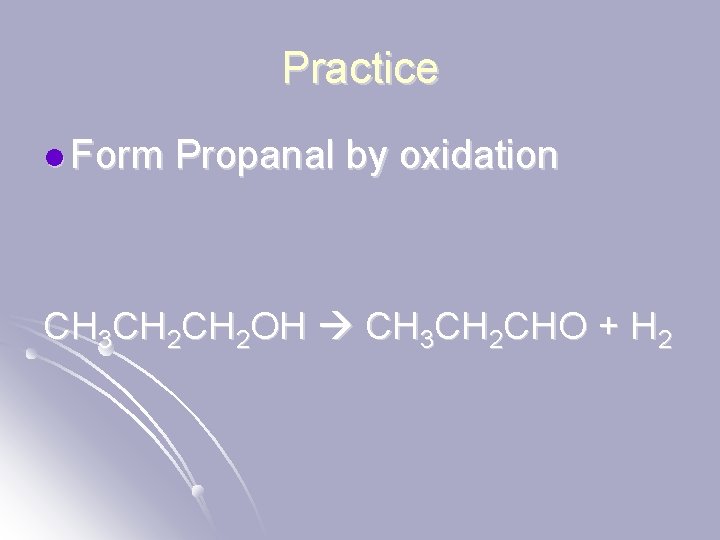
Practice l Form Propanal by oxidation CH 3 CH 2 OH CH 3 CH 2 CHO + H 2

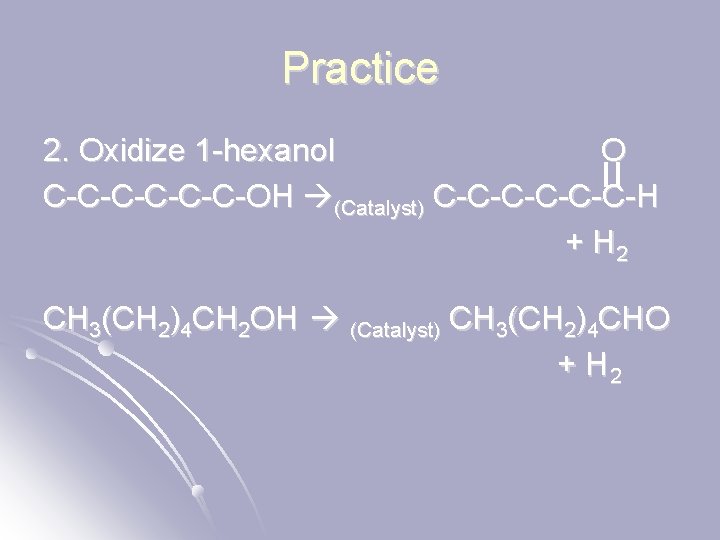
Practice 2. Oxidize 1 -hexanol O C-C-C-OH (Catalyst) C-C-C-H + H 2 CH 3(CH 2)4 CH 2 OH (Catalyst) CH 3(CH 2)4 CHO + H 2

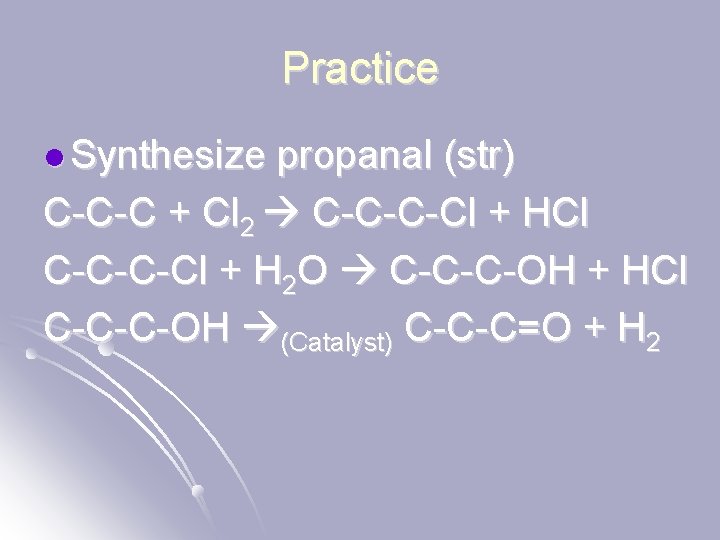
Practice l Synthesize propanal (str) C-C-C + Cl 2 C-C-C-Cl + HCl C-C-C-Cl + H 2 O C-C-C-OH + HCl C-C-C-OH (Catalyst) C-C-C=O + H 2

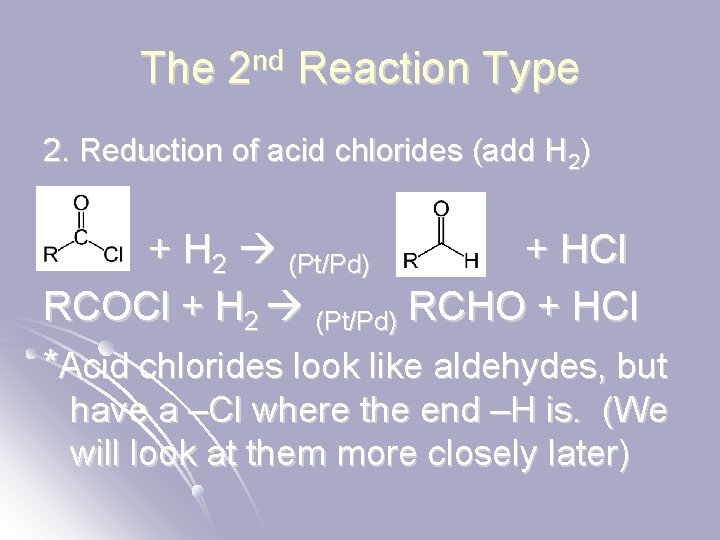
The 2 nd Reaction Type 2. Reduction of acid chlorides (add H 2) + H 2 (Pt/Pd) + HCl RCOCl + H 2 (Pt/Pd) RCHO + HCl *Acid chlorides look like aldehydes, but have a –Cl where the end –H is. (We will look at them more closely later)

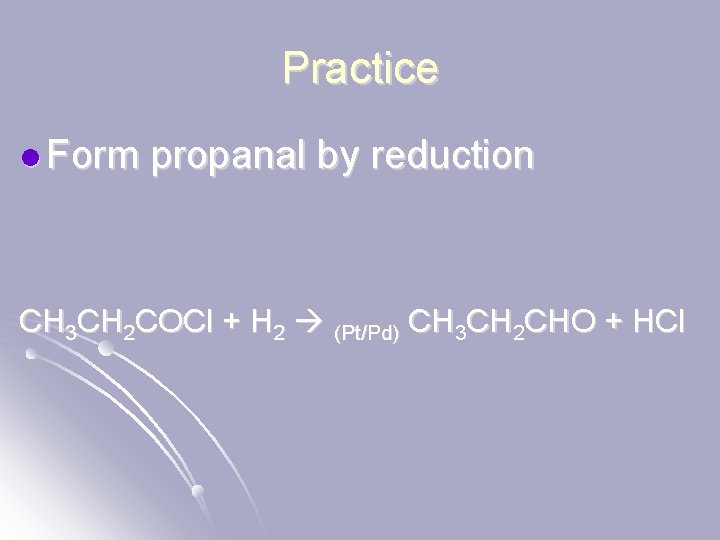
Practice l Form propanal by reduction CH 3 CH 2 COCl + H 2 (Pt/Pd) CH 3 CH 2 CHO + HCl

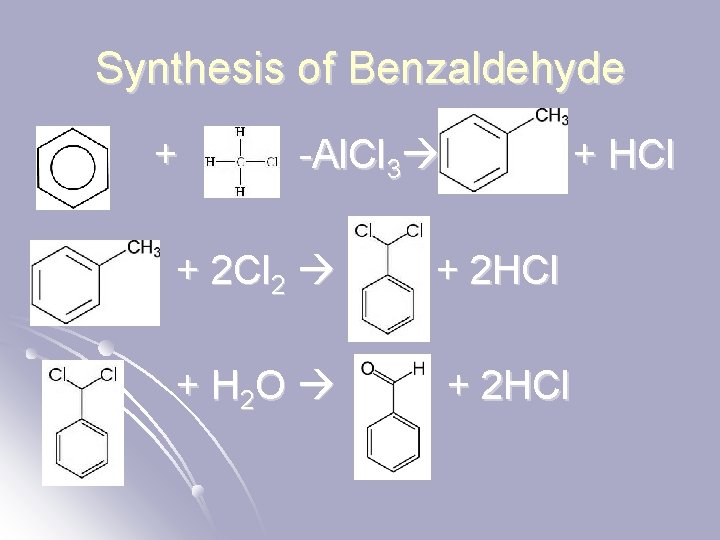
Synthesis of Benzaldehyde l The =O is not directly on the benzene, so you can’t follow he steps for an alcohol – you have to use the following…

Synthesis of Benzaldehyde + -Al. Cl 3 + HCl + 2 Cl 2 + 2 HCl + H 2 O + 2 HCl

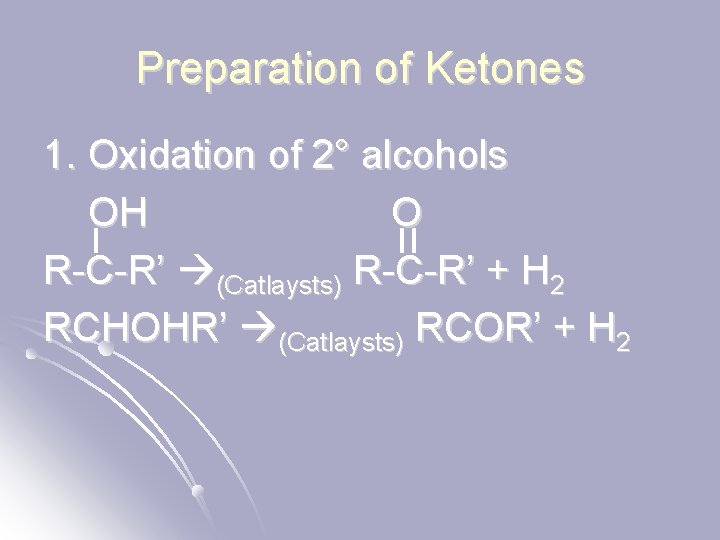
Preparation of Ketones 1. Oxidation of 2° alcohols OH O R-C-R’ (Catlaysts) R-C-R’ + H 2 RCHOHR’ (Catlaysts) RCOR’ + H 2

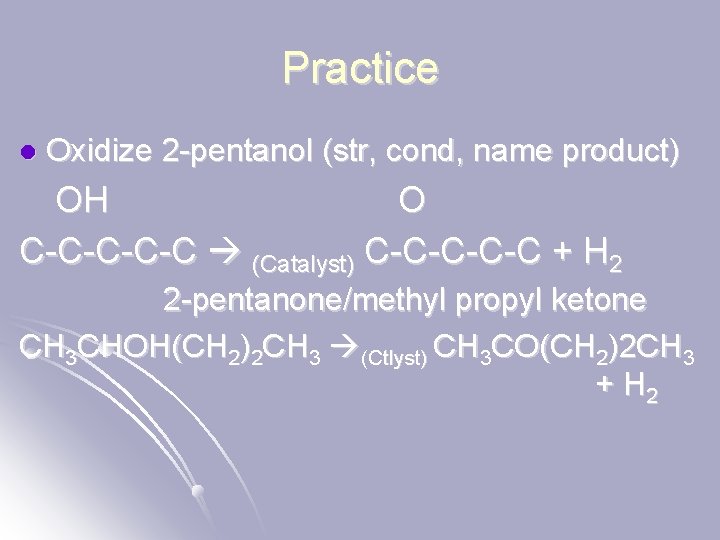
Practice l Oxidize 2 -pentanol (str, cond, name product) OH O C-C-C (Catalyst) C-C-C + H 2 2 -pentanone/methyl propyl ketone CH 3 CHOH(CH 2)2 CH 3 (Ctlyst) CH 3 CO(CH 2)2 CH 3 + H 2

Practice l Form methyl ketone (str, cond, name reactant)

Practice l Synthesize 3 -heptanone (cond. ) CH 3(CH 2)5 CH 3+Cl 2 CH 3 CH 2 CHCl(CH 2)3 CH 3 + HCl CH 3 CH 2 CHCl(CH 2)3 CH 3 + H 2 O CH 3 CH 2 CHOH(CH 2)3 CH 3 + HCl CH 3 CH 2 CHOH(CH 2)3 CH 3 (Cats) CH 3 CH 2 CO(CH 2)3 CH 3 + H 2

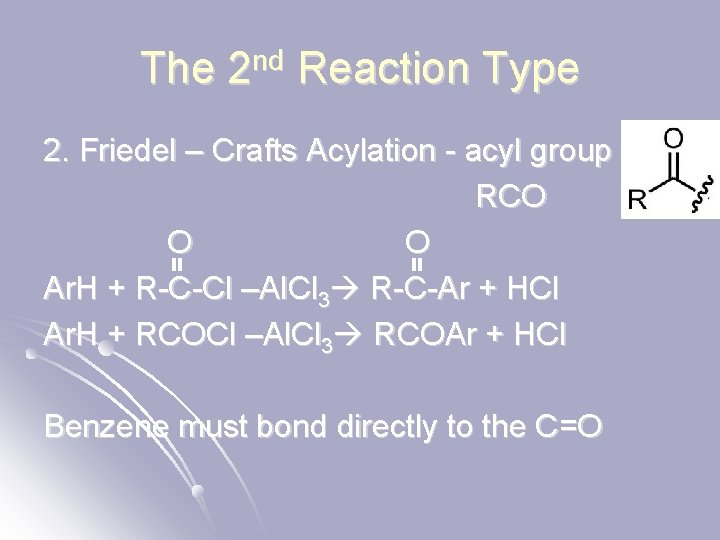
The 2 nd Reaction Type 2. Friedel – Crafts Acylation - acyl group RCO O O Ar. H + R-C-Cl –Al. Cl 3 R-C-Ar + HCl Ar. H + RCOCl –Al. Cl 3 RCOAr + HCl Benzene must bond directly to the C=O

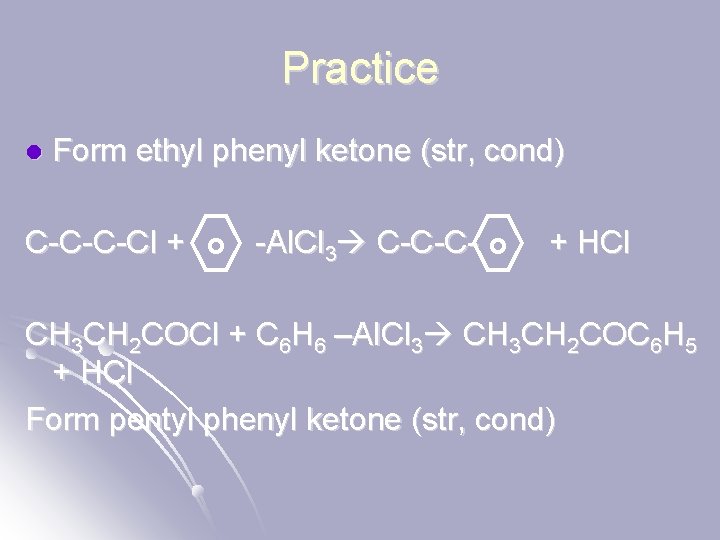
Practice l Form ethyl phenyl ketone (str, cond) C-C-C-Cl + -Al. Cl 3 C-C-C- + HCl CH 3 CH 2 COCl + C 6 H 6 –Al. Cl 3 CH 3 CH 2 COC 6 H 5 + HCl Form pentyl phenyl ketone (str, cond)

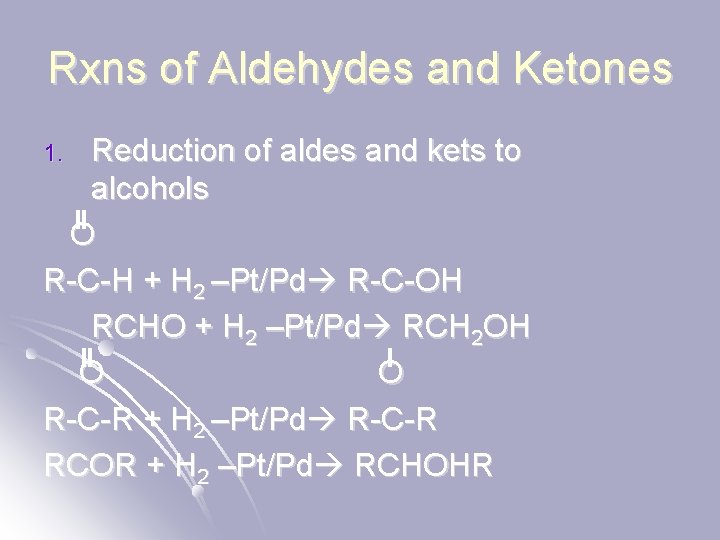
Rxns of Aldehydes and Ketones Reduction of aldes and kets to alcohols O R-C-H + H 2 –Pt/Pd R-C-OH RCHO + H 2 –Pt/Pd RCH 2 OH O O R-C-R + H 2 –Pt/Pd R-C-R RCOR + H 2 –Pt/Pd RCHOHR 1.

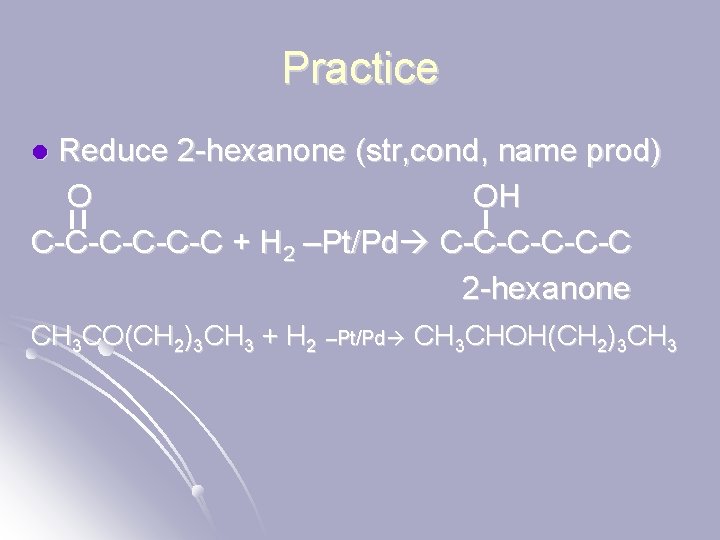
Practice Reduce 2 -hexanone (str, cond, name prod) O OH C-C-C-C + H 2 –Pt/Pd C-C-C-C 2 -hexanone l CH 3 CO(CH 2)3 CH 3 + H 2 –Pt/Pd CH 3 CHOH(CH 2)3 CH 3

Practice l Reduce Pentanal (str, cond, name prod)

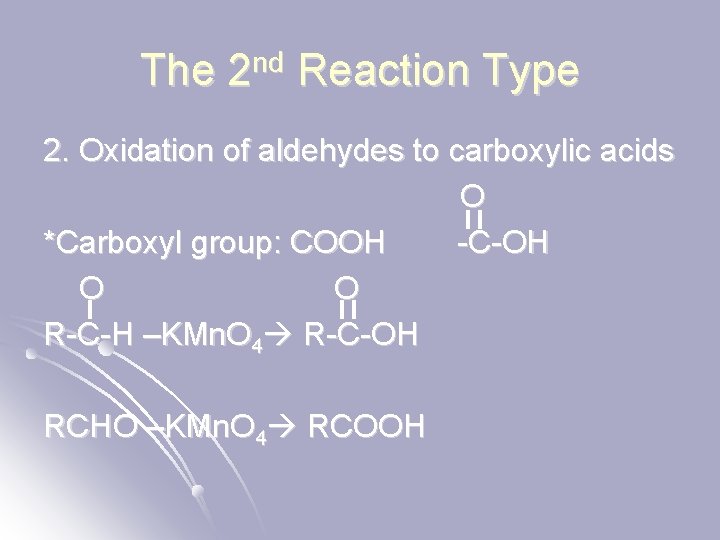
The 2 nd Reaction Type 2. Oxidation of aldehydes to carboxylic acids O *Carboxyl group: COOH -C-OH O O R-C-H –KMn. O 4 R-C-OH RCHO –KMn. O 4 RCOOH

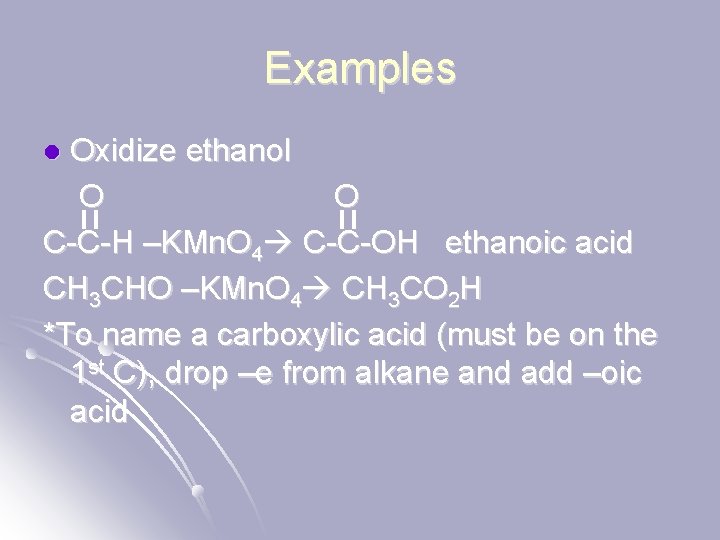
Examples Oxidize ethanol O O C-C-H –KMn. O 4 C-C-OH ethanoic acid CH 3 CHO –KMn. O 4 CH 3 CO 2 H *To name a carboxylic acid (must be on the 1 st C), drop –e from alkane and add –oic acid l

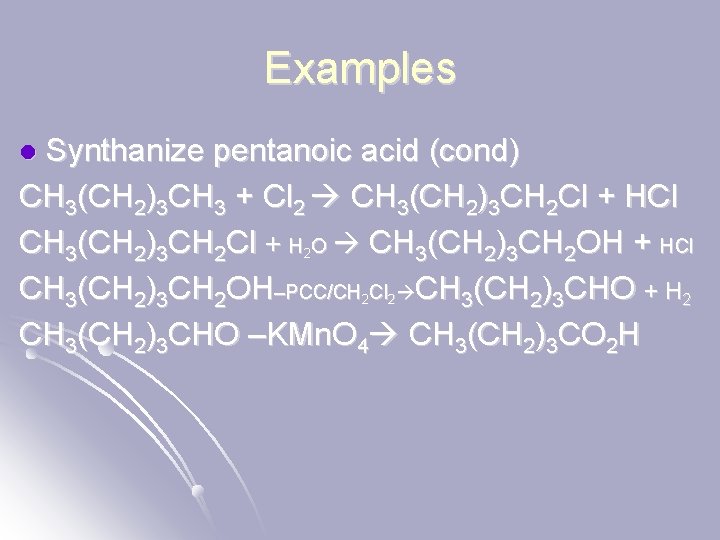
Examples Synthanize pentanoic acid (cond) CH 3(CH 2)3 CH 3 + Cl 2 CH 3(CH 2)3 CH 2 Cl + HCl CH 3(CH 2)3 CH 2 Cl + H 2 O CH 3(CH 2)3 CH 2 OH + HCl CH 3(CH 2)3 CH 2 OH–PCC/CH 2 Cl 2 CH 3(CH 2)3 CHO + H 2 CH 3(CH 2)3 CHO –KMn. O 4 CH 3(CH 2)3 CO 2 H l

3 RD Reaction Type Rxn with alcohols to form hemiacetals and acetals *Works well with aldehydes but difficule with ketones l

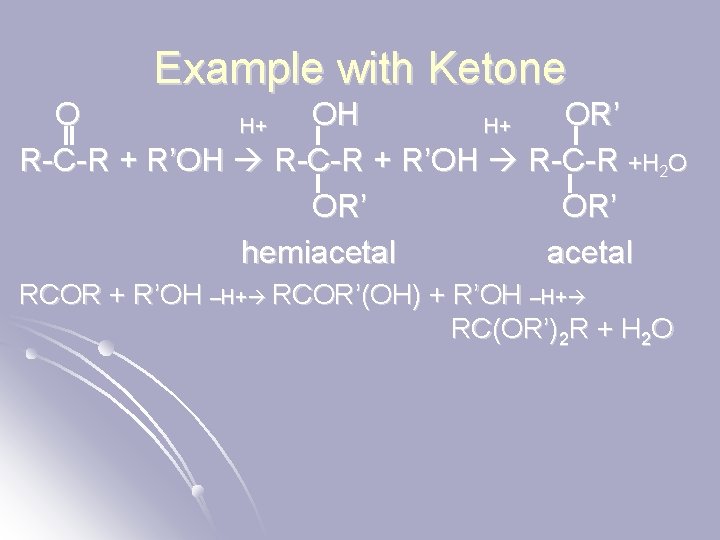
Example with Ketone O OH OR’ H+ H+ R-C-R + R’OH R-C-R +H 2 O OR’ hemiacetal RCOR + R’OH –H+ RCOR’(OH) + R’OH –H+ RC(OR’)2 R + H 2 O

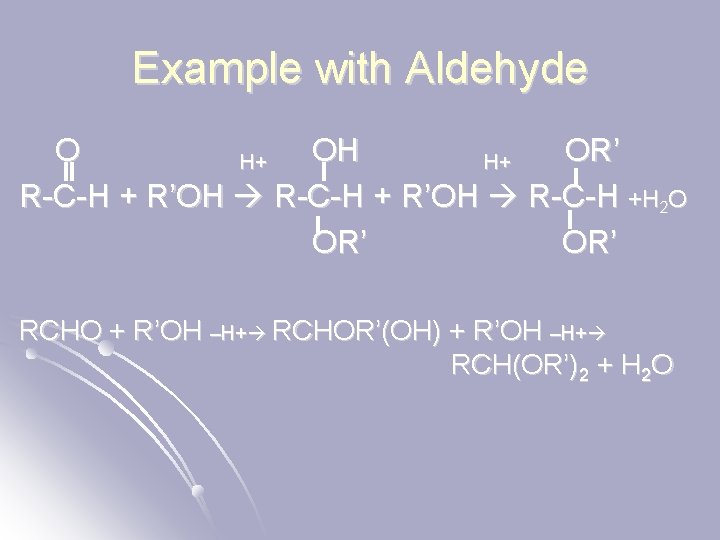
Example with Aldehyde O OH OR’ H+ H+ R-C-H + R’OH R-C-H +H 2 O OR’ RCHO + R’OH –H+ RCHOR’(OH) + R’OH –H+ RCH(OR’)2 + H 2 O

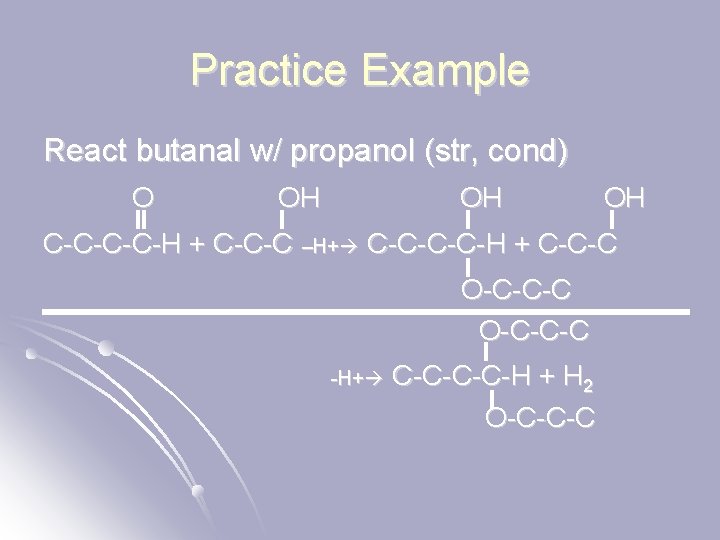
Practice Example React butanal w/ propanol (str, cond) O OH OH OH C-C-H + C-C-C –H+ C-C-H + C-C-C O-C-C-C -H+ C-C-H + H 2 O-C-C-C

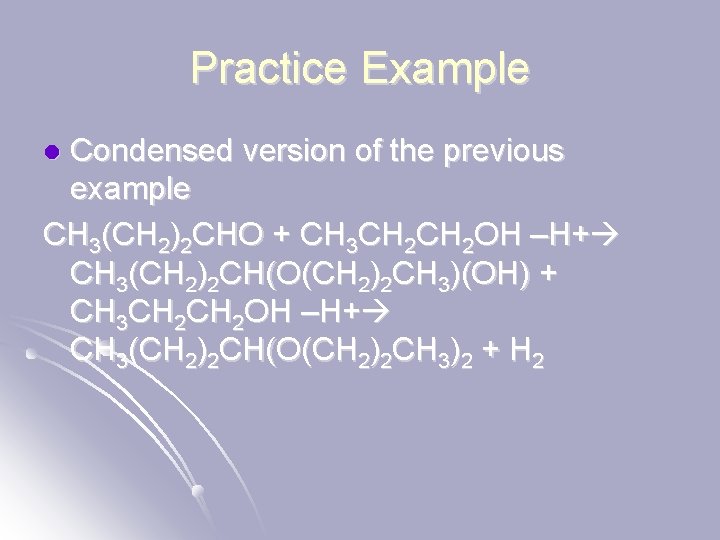
Practice Example Condensed version of the previous example CH 3(CH 2)2 CHO + CH 3 CH 2 OH –H+ CH 3(CH 2)2 CH(O(CH 2)2 CH 3)(OH) + CH 3 CH 2 OH –H+ CH 3(CH 2)2 CH(O(CH 2)2 CH 3)2 + H 2 l

Practice l React 2 -pentanone with ethanol (str, cond)