ALDEHYDES AND KETONES Aldehyde Ketone STRUCTURE NOMENCLATURE IUPAC













- Slides: 48

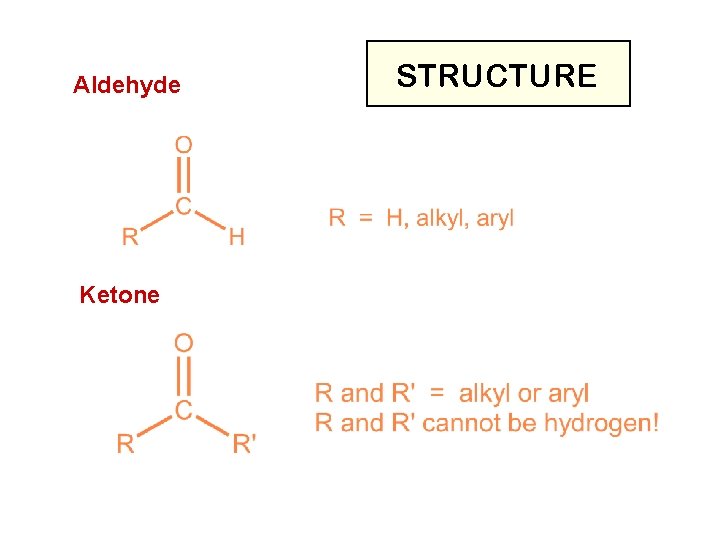
ALDEHYDES AND KETONES

Aldehyde Ketone STRUCTURE

NOMENCLATURE

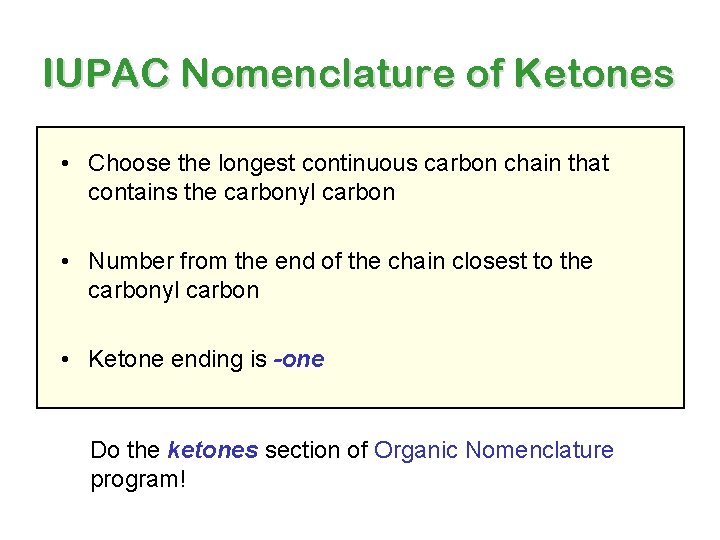
IUPAC Nomenclature of Ketones • Choose the longest continuous carbon chain that contains the carbonyl carbon • Number from the end of the chain closest to the carbonyl carbon • Ketone ending is -one Do the ketones section of Organic Nomenclature program!

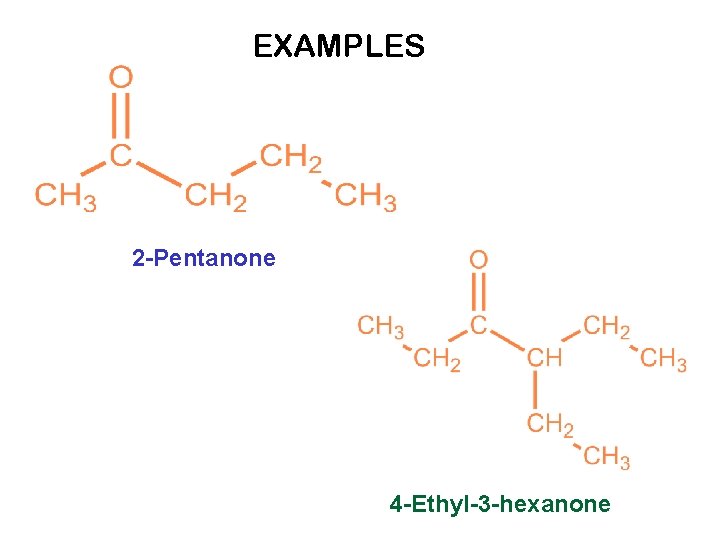
EXAMPLES 2 -Pentanone 4 -Ethyl-3 -hexanone

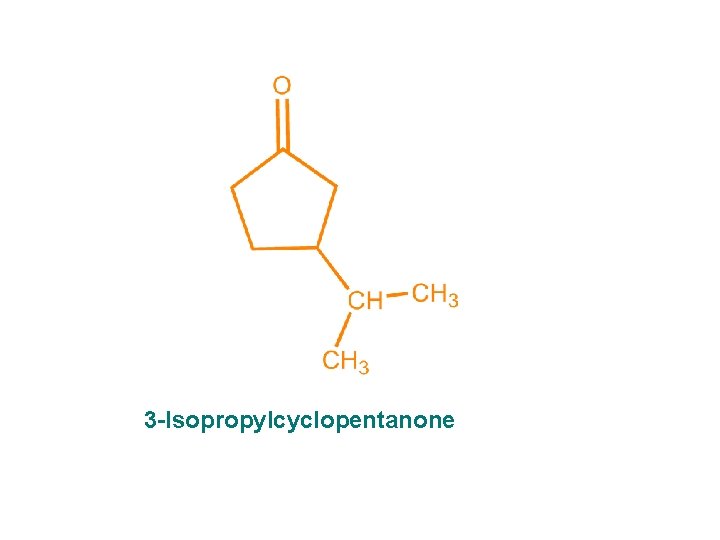
3 -Isopropylcyclopentanone

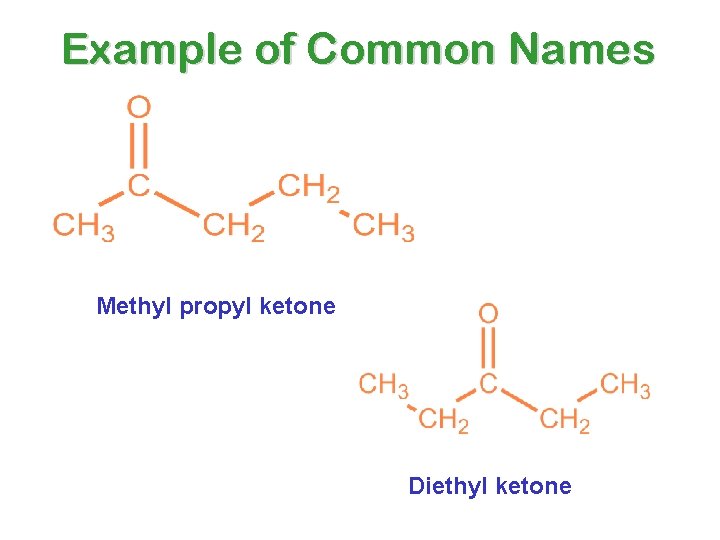
KETONES Common, or Trivial, Names • Name each group attached to the carbonyl group as an alkyl group • Combine into a name, according to the pattern: alkyl’ ketone NOTE: This is not all one word!

Example of Common Names Methyl propyl ketone Diethyl ketone

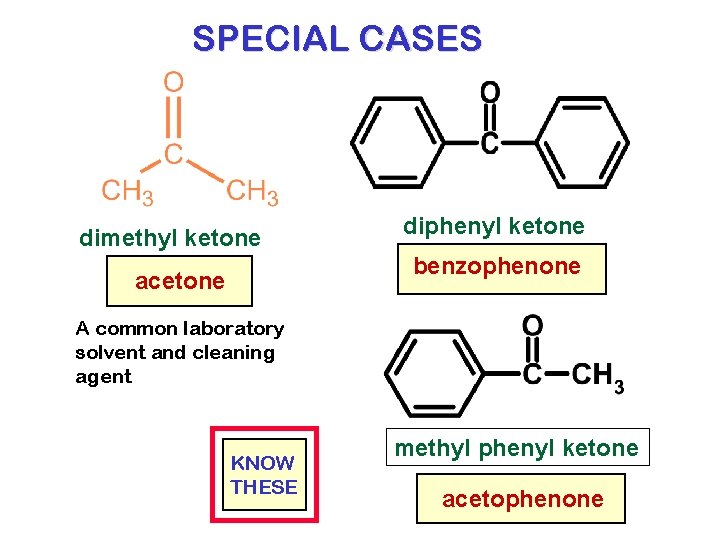
SPECIAL CASES dimethyl ketone diphenyl ketone benzophenone acetone A common laboratory solvent and cleaning agent KNOW THESE methyl phenyl ketone acetophenone

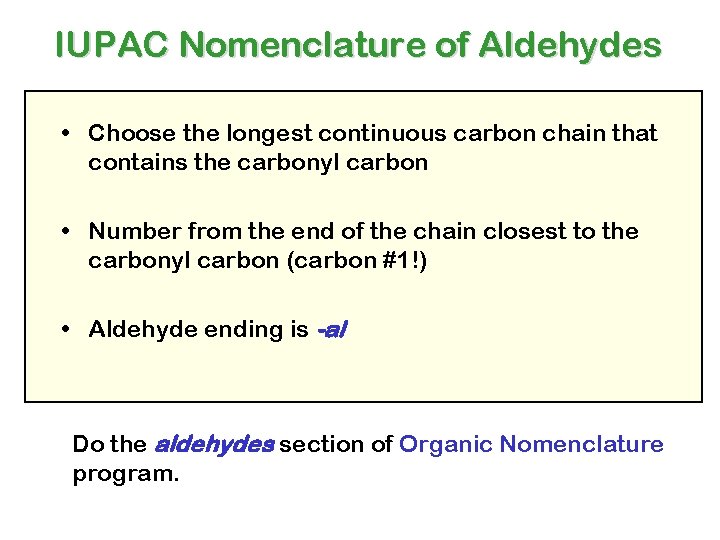
IUPAC Nomenclature of Aldehydes • Choose the longest continuous carbon chain that contains the carbonyl carbon • Number from the end of the chain closest to the carbonyl carbon (carbon #1!) • Aldehyde ending is -al Do the aldehydes section of Organic Nomenclature program.

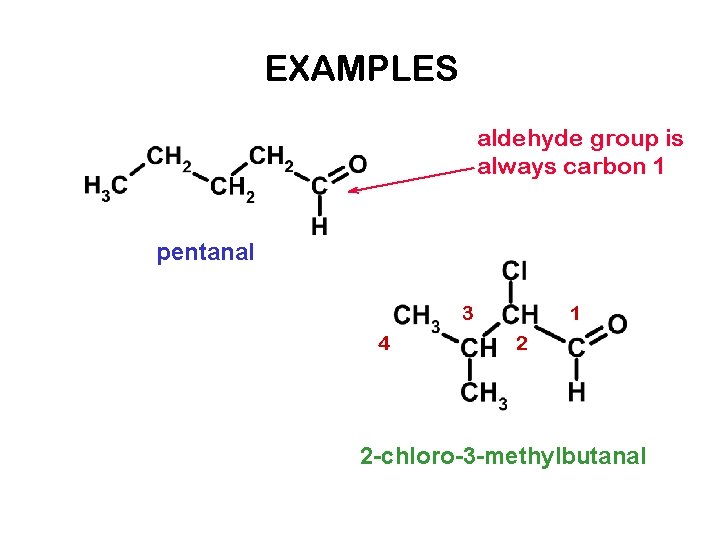
EXAMPLES aldehyde group is always carbon 1 pentanal 3 4 1 2 2 -chloro-3 -methylbutanal

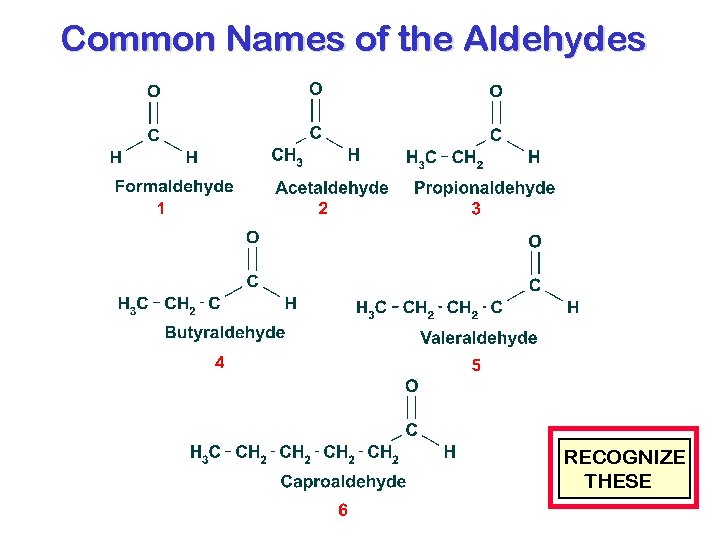
Common Names of the Aldehydes RECOGNIZE THESE

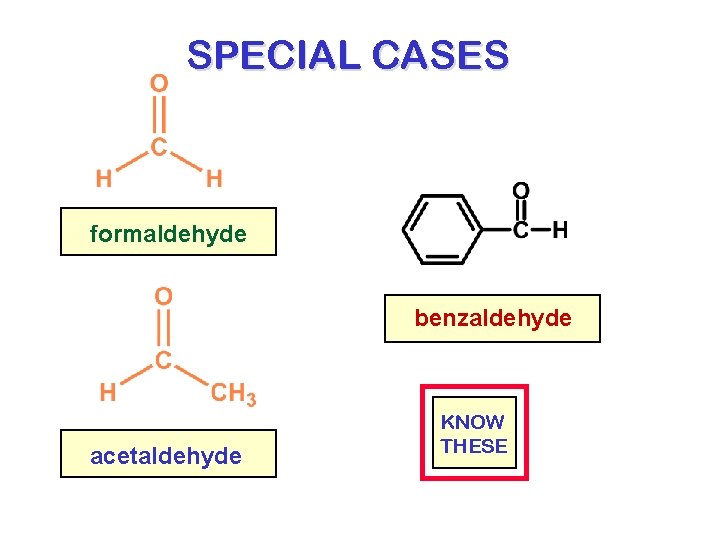
SPECIAL CASES formaldehyde benzaldehyde acetaldehyde KNOW THESE

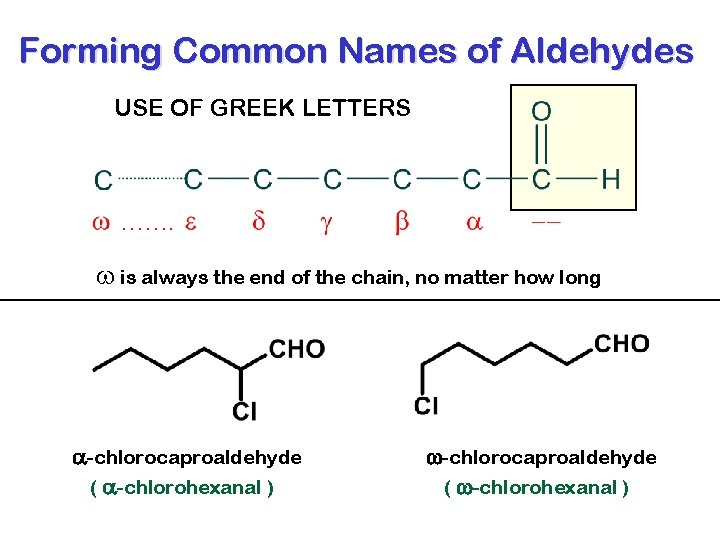
Forming Common Names of Aldehydes USE OF GREEK LETTERS ……. w is always the end of the chain, no matter how long a-chlorocaproaldehyde ( a-chlorohexanal ) w-chlorocaproaldehyde ( w-chlorohexanal )

REACTIVITY OF THE C=O GROUP NUCLEOPHILIC ADDITION

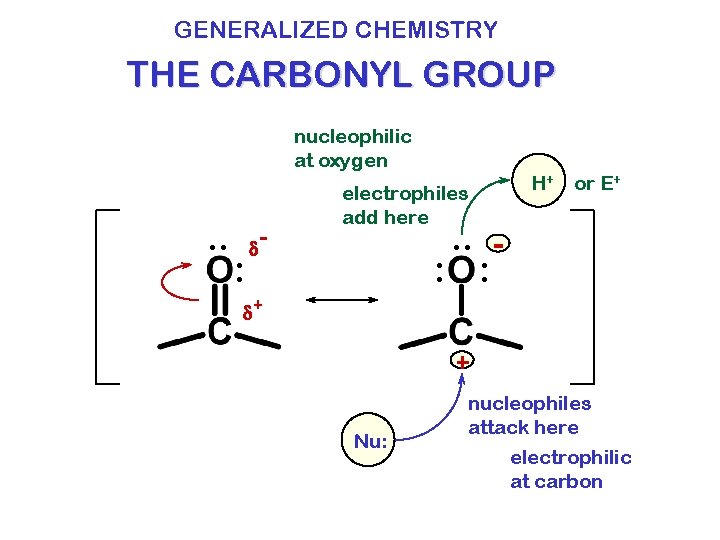
GENERALIZED CHEMISTRY THE CARBONYL GROUP nucleophilic at oxygen . . : d - electrophiles add here H+ or E+ . . : : d+ + Nu: nucleophiles attack here electrophilic at carbon

NUCLEOPHILIC ADDITION TO C=O MECHANISMS IN ACID AND IN BASE

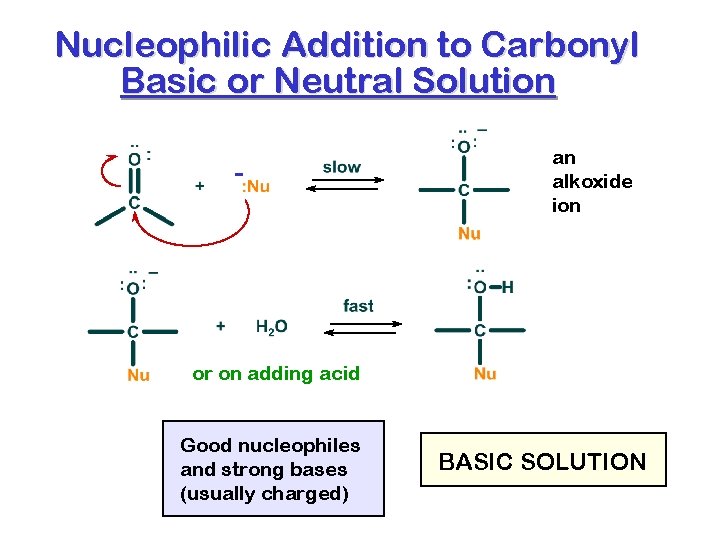
Nucleophilic Addition to Carbonyl Basic or Neutral Solution - an alkoxide ion or on adding acid Good nucleophiles and strong bases (usually charged) BASIC SOLUTION

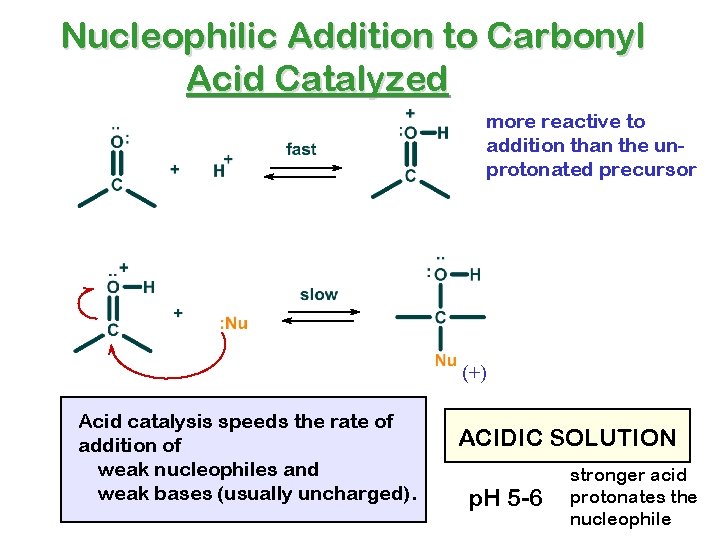
Nucleophilic Addition to Carbonyl Acid Catalyzed more reactive to addition than the unprotonated precursor (+) Acid catalysis speeds the rate of addition of weak nucleophiles and weak bases (usually uncharged). ACIDIC SOLUTION p. H 5 -6 stronger acid protonates the nucleophile

CYANOHYDRINS

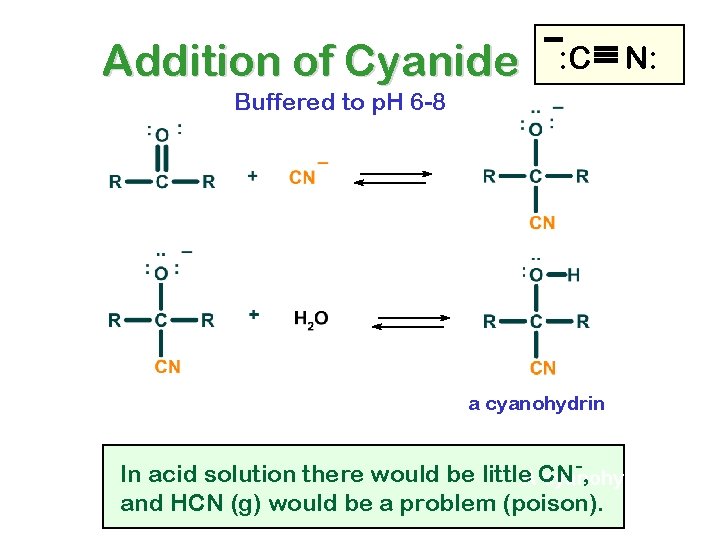
Addition of Cyanide : C N: Buffered to p. H 6 -8 a cyanohydrin -, In acid solution there would be little. ACN cyanohydrin and HCN (g) would be a problem (poison).

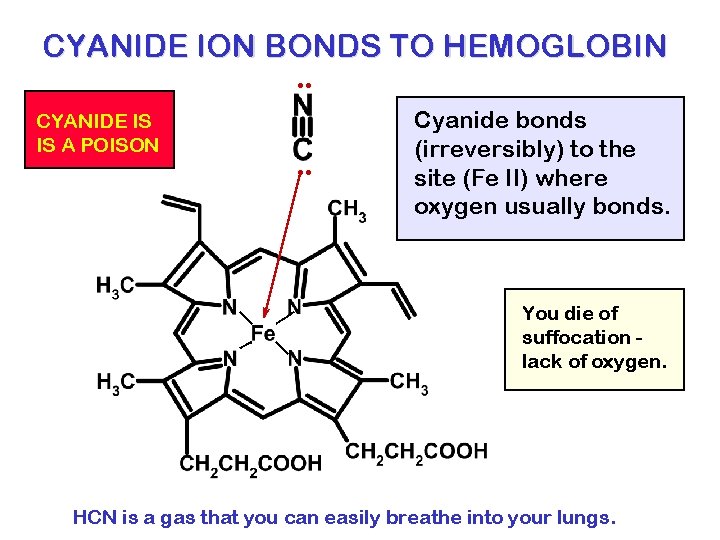
CYANIDE ION BONDS TO HEMOGLOBIN. . CYANIDE IS IS A POISON . . Cyanide bonds (irreversibly) to the site (Fe II) where oxygen usually bonds. You die of suffocation lack of oxygen. HCN is a gas that you can easily breathe into your lungs.

ORGANOMETALLICS

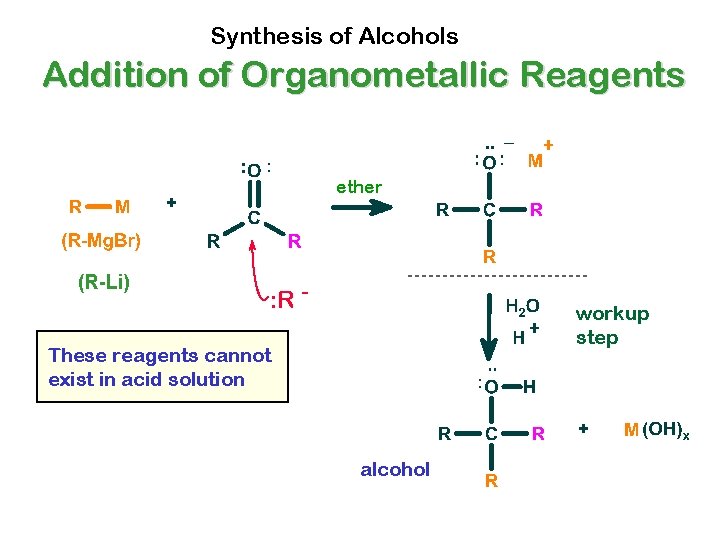
Synthesis of Alcohols Addition of Organometallic Reagents ether (R-Li) : R - workup step These reagents cannot exist in acid solution alcohol

Summary of Reactions of Organometallics with Carbonyl Compounds All review to you • Organometallics with ketones yield tertiary alcohols • Organometallics with aldehydes yield secondary alcohols • Organometallics with formaldehyde yield primary alcohols. • Organometallics with carbon dioxide yield carboxylic acids. etc.

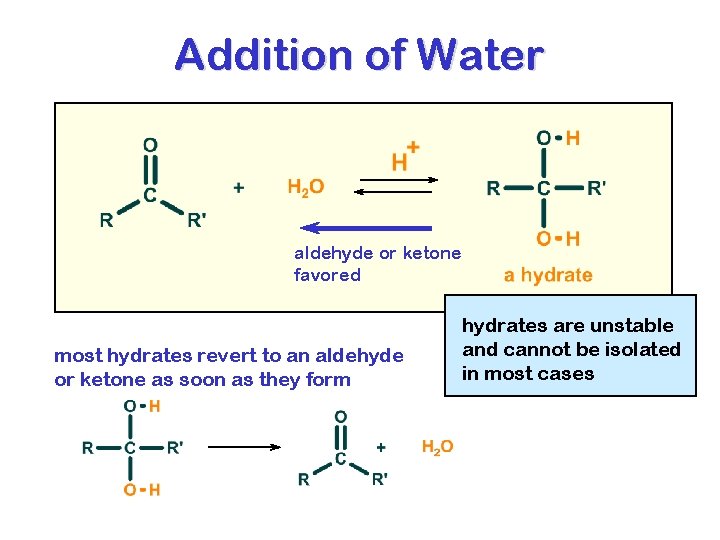
HYDRATES

Addition of Water aldehyde or ketone favored most hydrates revert to an aldehyde or ketone as soon as they form hydrates are unstable and cannot be isolated in most cases

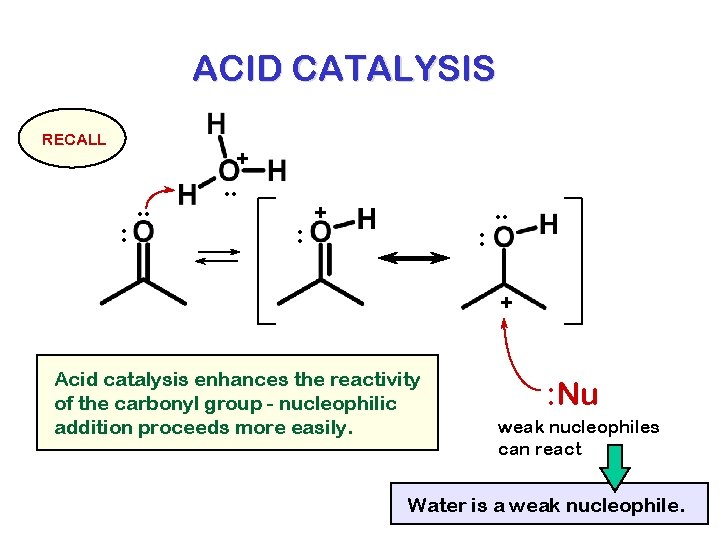
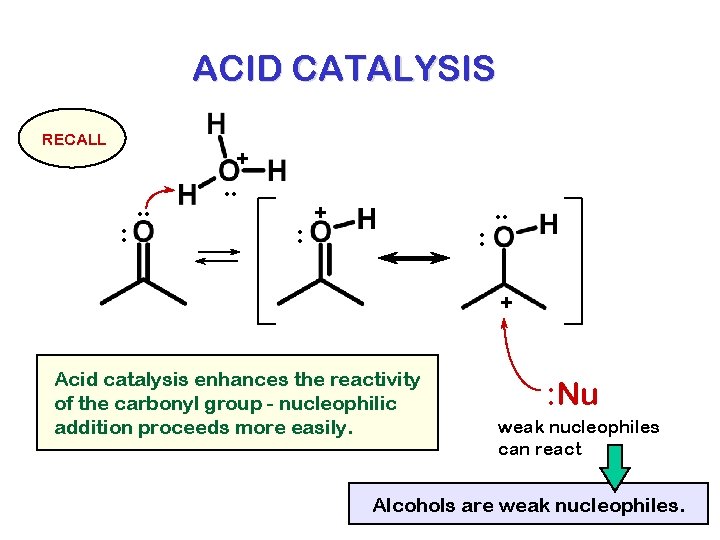
ACID CATALYSIS RECALL + : . . : + : . . + Acid catalysis enhances the reactivity of the carbonyl group - nucleophilic addition proceeds more easily. : Nu weak nucleophiles can react Water is a weak nucleophile.

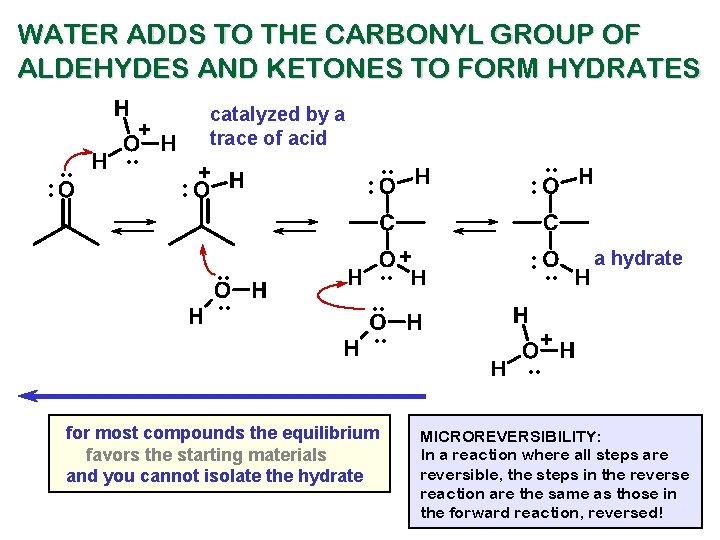
WATER ADDS TO THE CARBONYL GROUP OF ALDEHYDES AND KETONES TO FORM HYDRATES catalyzed by a trace of acid + : . . : + : . . . for most compounds the equilibrium favors the starting materials and you cannot isolate the hydrate . . : + : . . a hydrate + . . MICROREVERSIBILITY: In a reaction where all steps are reversible, the steps in the reverse reaction are the same as those in the forward reaction, reversed!

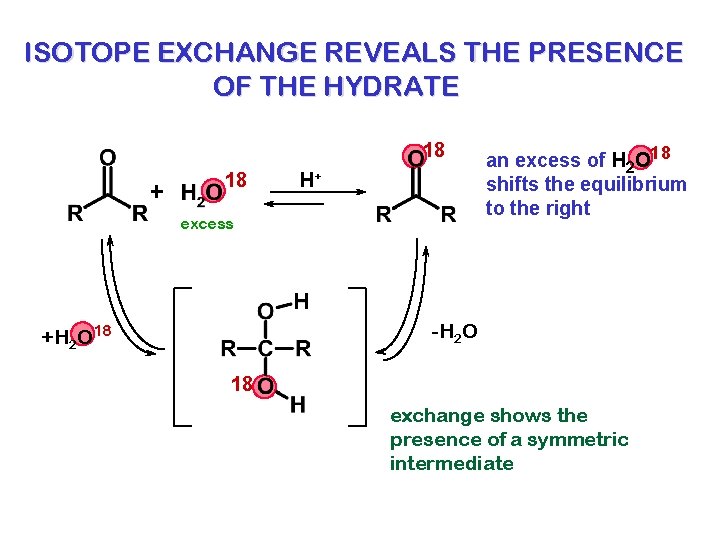
ISOTOPE EXCHANGE REVEALS THE PRESENCE OF THE HYDRATE 18 + 18 H+ excess an excess of H 2 O 18 shifts the equilibrium to the right -H 2 O +H 2 O 18 18 exchange shows the presence of a symmetric intermediate

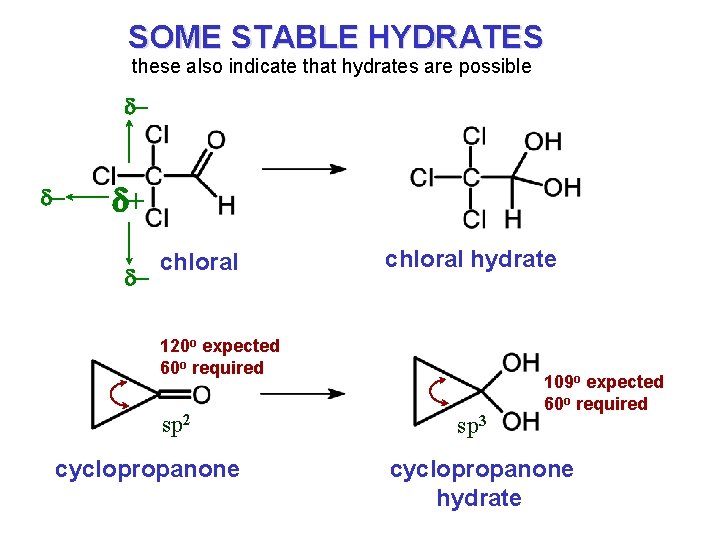
SOME STABLE HYDRATES these also indicate that hydrates are possible d- d- d+ d- chloral hydrate 120 o expected 60 o required sp 2 cyclopropanone sp 3 109 o expected 60 o required cyclopropanone hydrate

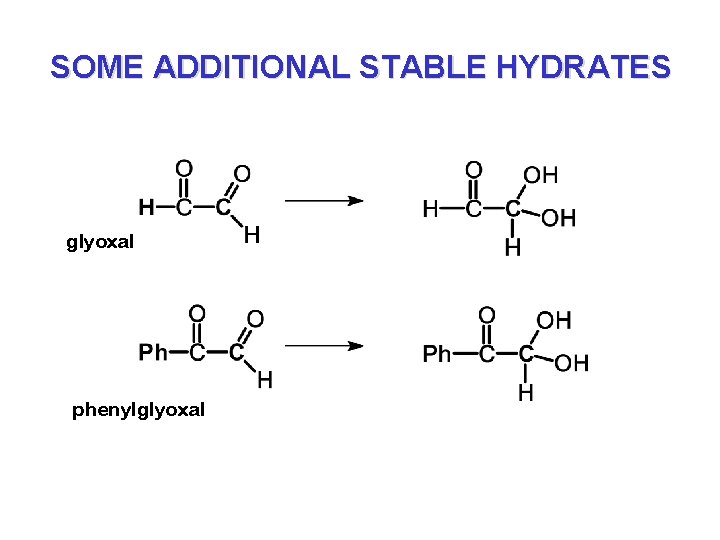
SOME ADDITIONAL STABLE HYDRATES glyoxal phenylglyoxal

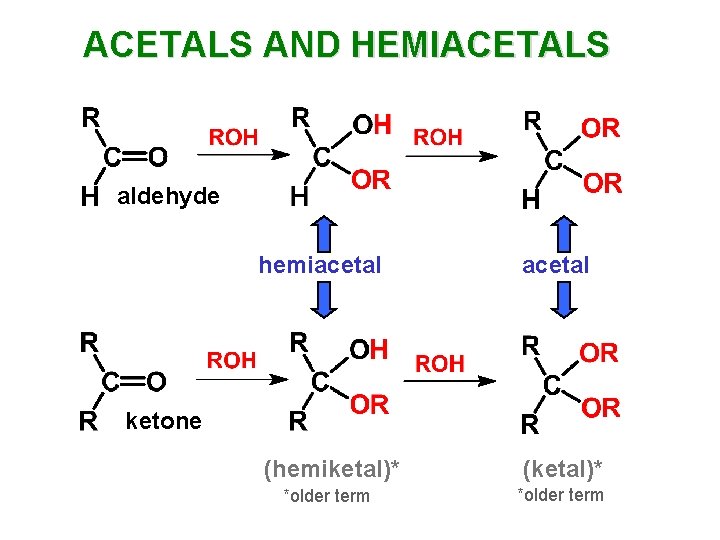
ACETALS AND HEMIACETALS

ACID CATALYSIS RECALL + : . . : + : . . + Acid catalysis enhances the reactivity of the carbonyl group - nucleophilic addition proceeds more easily. : Nu weak nucleophiles can react Alcohols are weak nucleophiles.

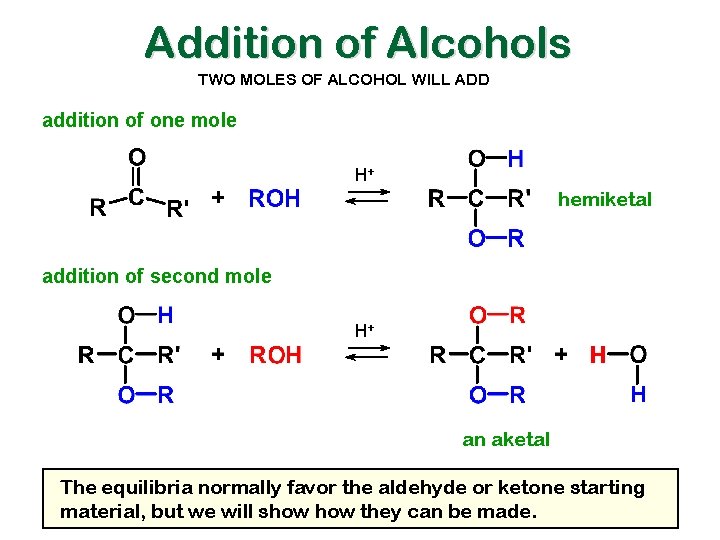
Addition of Alcohols TWO MOLES OF ALCOHOL WILL ADD addition of one mole H+ hemiketal addition of second mole H+ an aketal The equilibria normally favor the aldehyde or ketone starting material, but we will show they can be made.

ACETALS AND HEMIACETALS aldehyde hemiacetal (hemiketal)* (ketal)* *older term ketone

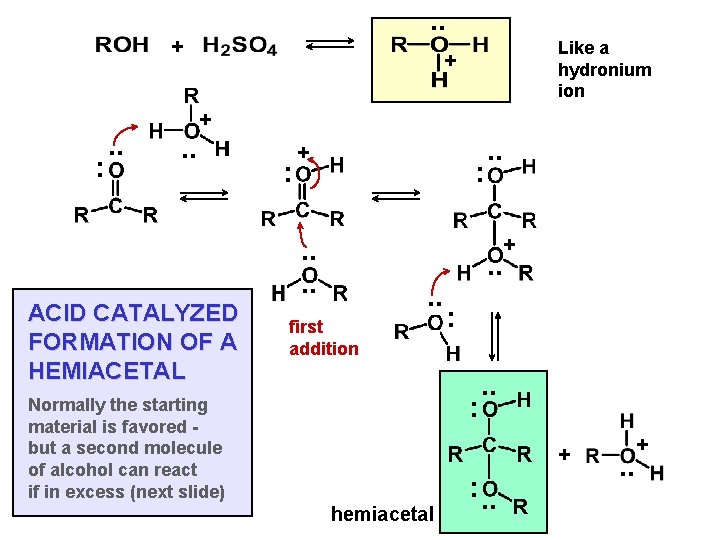
. . + : . . Like a hydronium ion + + . . ACID CATALYZED FORMATION OF A HEMIACETAL : + : . . + . . . . first addition : : Normally the starting material is favored but a second molecule of alcohol can react if in excess (next slide) . . + hemiacetal : . . +

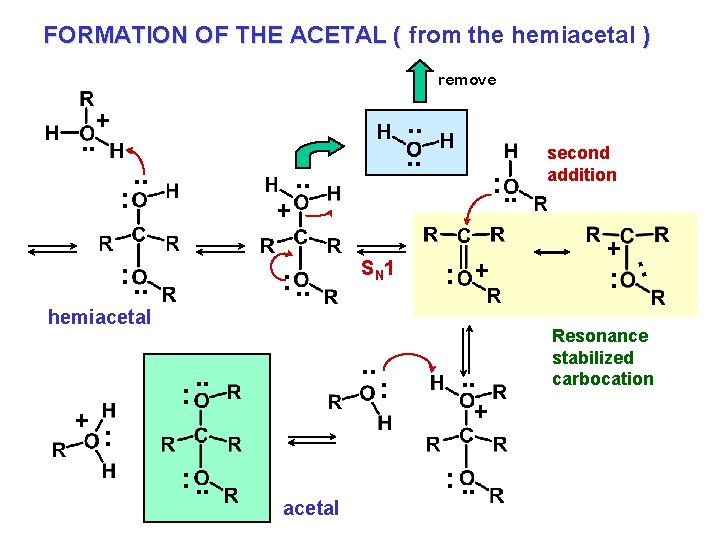
FORMATION OF THE ACETAL ( from the hemiacetal ) remove + . . . : . . + : . . SN 1 : + hemiacetal + : . . : : . . acetal : . . + : . . second addition + : : Resonance stabilized carbocation

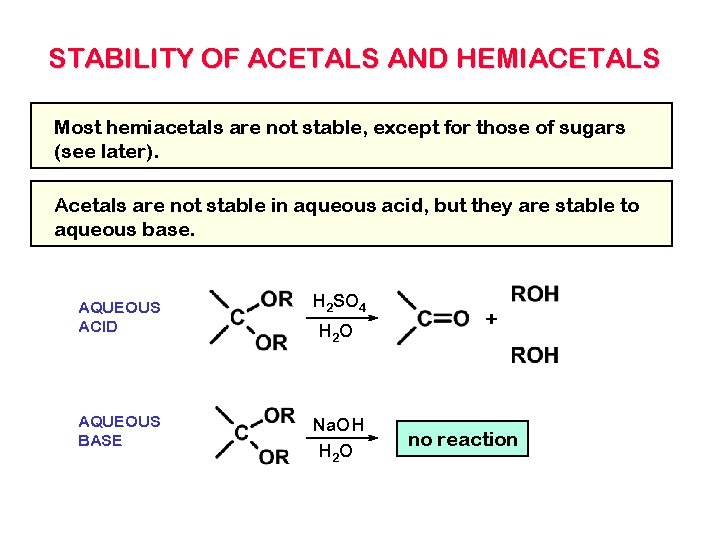
STABILITY OF ACETALS AND HEMIACETALS Most hemiacetals are not stable, except for those of sugars (see later). Acetals are not stable in aqueous acid, but they are stable to aqueous base. AQUEOUS ACID H 2 SO 4 AQUEOUS BASE Na. OH H 2 O + no reaction

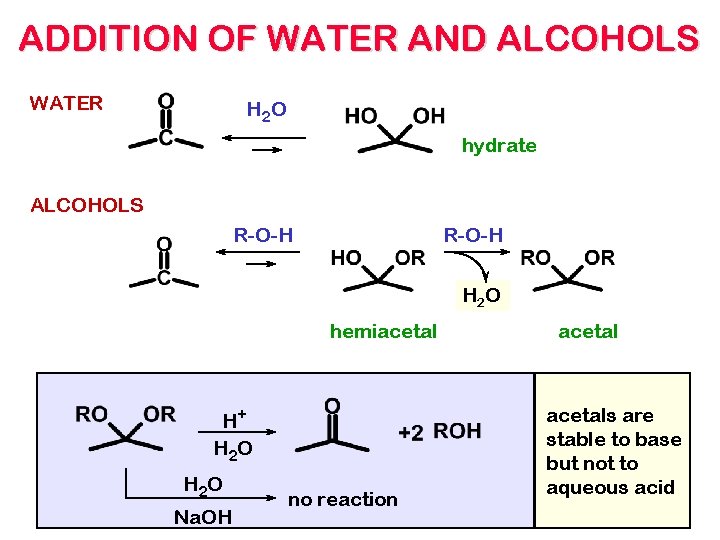
ADDITION OF WATER AND ALCOHOLS WATER H 2 O hydrate ALCOHOLS R-O-H H 2 O hemiacetal H+ H 2 O Na. OH no reaction acetals are stable to base but not to aqueous acid

REAKSI OKSIDASI

OKSIDASI ALDEHID DAN KETON • Keton tidak mudah dioksidasi • Aldehid sangat mudah dioksidasi, menjadi asam karboksilat Zat pengoksidasi : KMn. O 4, H, H 2 O

Reaksi Reduksi

Reaksi Reduksi • Reduksi aldehid menghasilkan alkohol primer • Reduksi keton menghasilkan alkohol sekunder • Zat pereduksi: H 2, katalis Zn/Hg, HCl

Reaksi Adisi-eliminasi

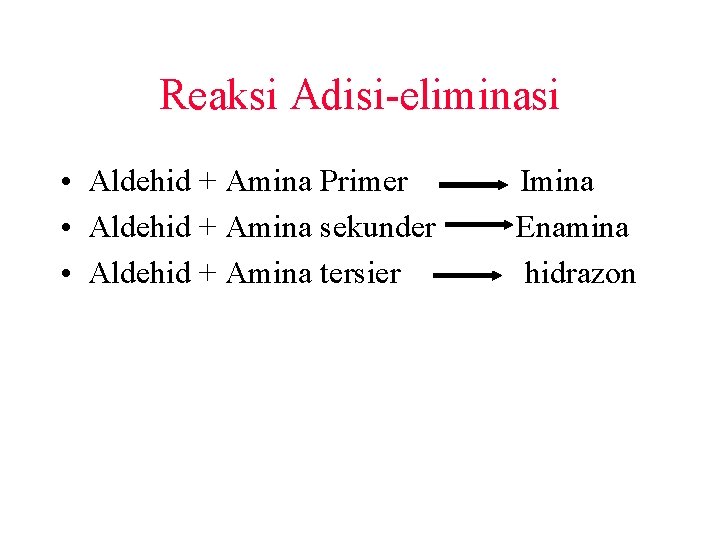
Reaksi Adisi-eliminasi • Aldehid + Amina Primer • Aldehid + Amina sekunder • Aldehid + Amina tersier Imina Enamina hidrazon

Ramalkan produk hemiasetal atau hemiasetal siklik dari: 1. 5 -hidroksi-2 -heksanon dengan air 2. 1, 3, 4, 5, 6 -pentahidroksi-2 -heksanon dengan air 3. propanal dengan metanol 4. Aseton dengan 1, 2, 3 -propanatriol

Ramalkan apa produk reaksi sikloheksanon dengan : 1. CH 3 NH 2 2. (CH 3)2 NH