Alcohols and Ethers Section 1 4 Alcohols Alcohol





- Slides: 13

Alcohols and Ethers Section 1. 4

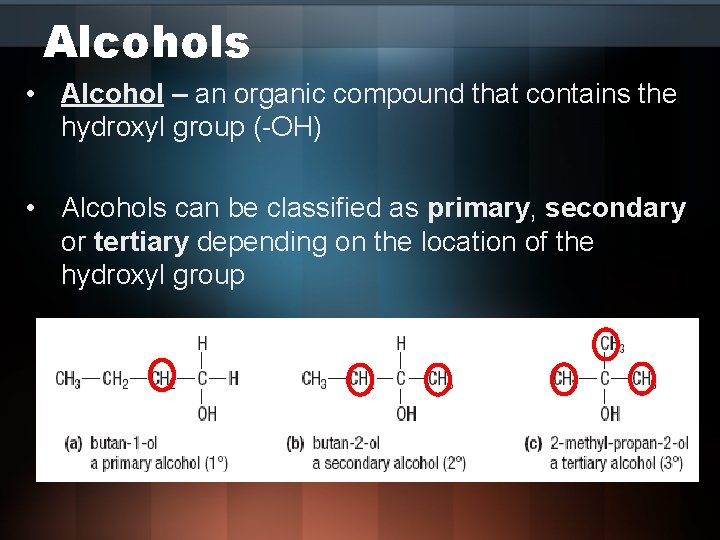
Alcohols • Alcohol – an organic compound that contains the hydroxyl group (-OH) • Alcohols can be classified as primary, secondary or tertiary depending on the location of the hydroxyl group

Naming Alcohols • Name an alcohol by replacing the final “e” of the parent hydrocarbon with “ol” • The name must include the number of the carbon atom to which the hydroxyl group is attached. • If the chain also has hydrocarbon or halide branches, we assign the lowest number to the carbon atom with the hydroxyl group and place this at the front. *Hydroxyl group will get the priority* • Use the words diol or triol for multiple OH groups.

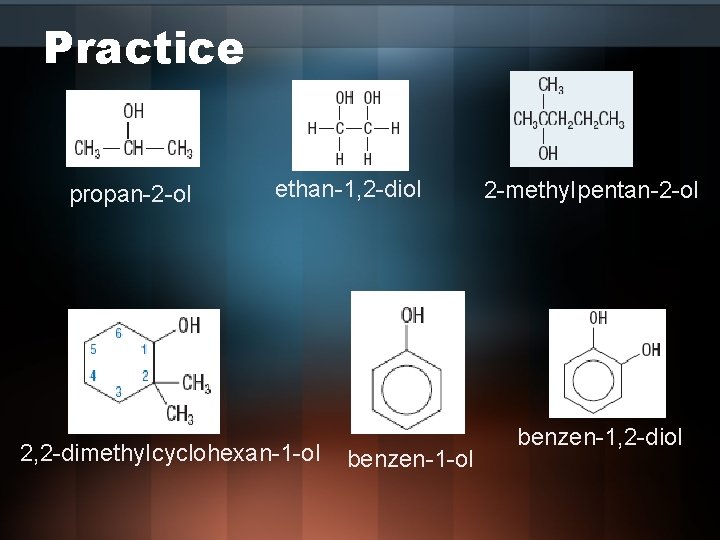
Practice propan-2 -ol ethan-1, 2 -diol 2, 2 -dimethyl cyclohexan-1 -ol benzen-1 -ol 2 -methyl pentan-2 -ol benzen-1, 2 -diol

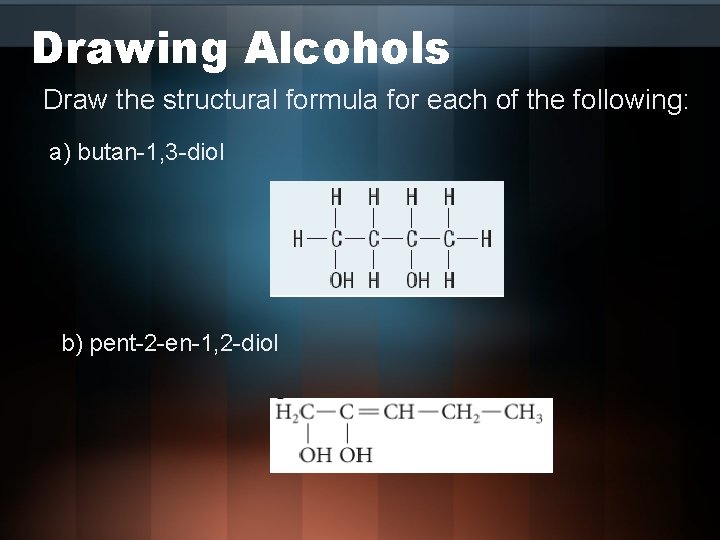
Drawing Alcohols Draw the structural formula for each of the following: a) butan-1, 3 -diol b) pent-2 -en-1, 2 -diol

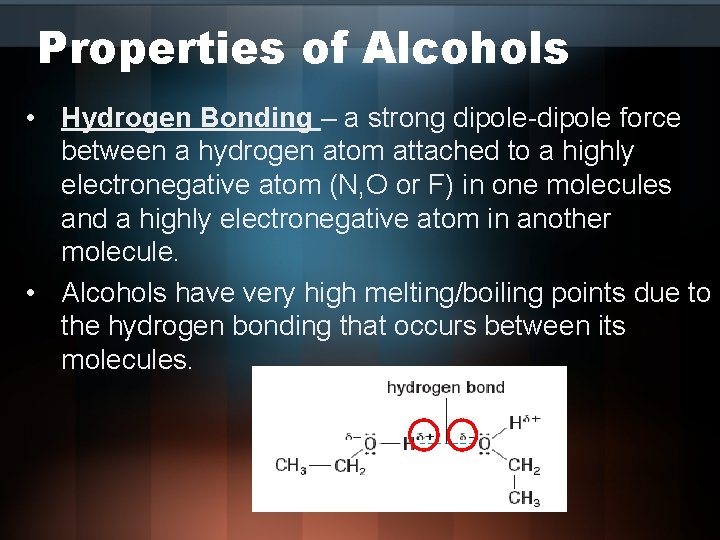
Properties of Alcohols • Hydrogen Bonding – a strong dipole-dipole force between a hydrogen atom attached to a highly electronegative atom (N, O or F) in one molecules and a highly electronegative atom in another molecule. • Alcohols have very high melting/boiling points due to the hydrogen bonding that occurs between its molecules.

Properties of Alcohols • Short carbon chained alcohols are more soluble in water than those with longer carbon chains. • The addition of the –OH group increases the polarity of the alcohol molecule and therefore its solubility in water, however as the chain grows, alcohols become less soluble. CH 3 OH - Methanol CH 3 CH 2 CH 2 CH 2 OH - Heptanol Nonpolar chain *ANY LONGER THAN 5 CARBONS WITH ONLY 1 ALCOHOL IT BECOMES NONPOLAR

Ethers • Ether – an organic compound that contains a functional group in which an oxygen atom is bonded between 2 carbon atoms within a chain. Naming Ethers • Small hydrocarbon“oxy” larger hydrocarbon • Put a number in front of the name to indicate which carbon the larger hydrocarbon is attached to the oxygen. • Ex. 1 - methoxypropane

Properties of Ethers • Ethers contain C-O bonds which makes the molecule polar. This means that ethers have boiling points slightly higher than those of similar-sized alkanes but lower than alcohols.

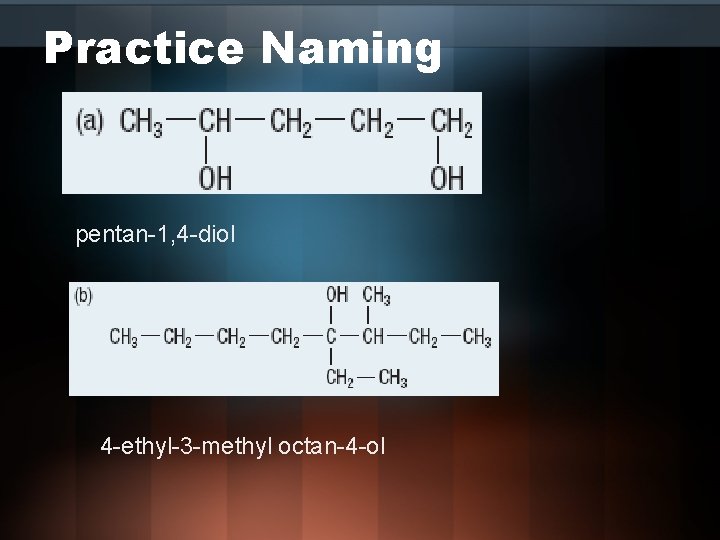
Practice Naming pentan-1, 4 -diol 4 -ethyl-3 -methyl octan-4 -ol

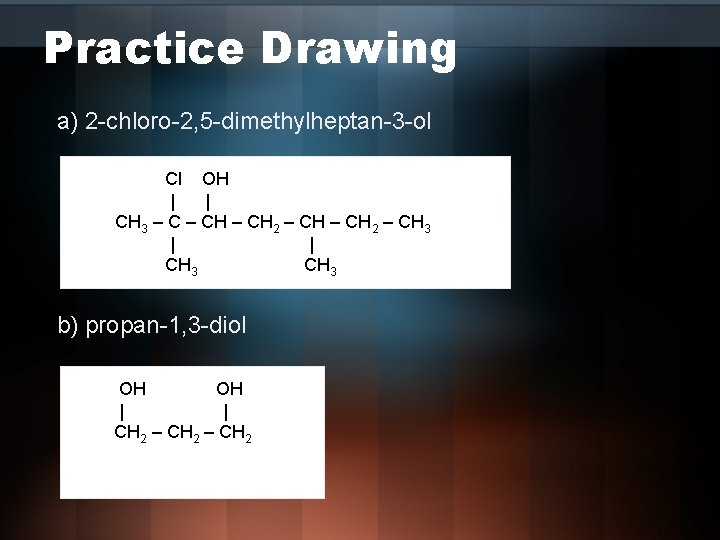
Practice Drawing a) 2 -chloro-2, 5 -dimethylheptan-3 -ol Cl OH | | CH 3 – CH 2 – CH 3 | | CH 3 b) propan-1, 3 -diol OH OH | | CH 2 – CH 2

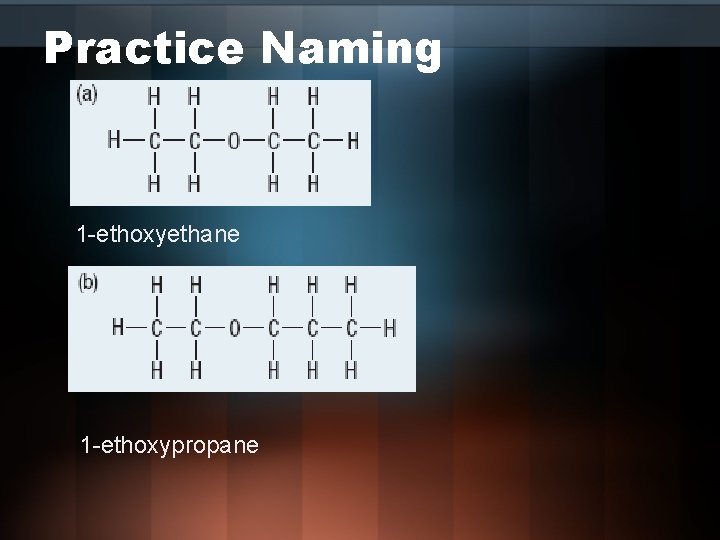
Practice Naming 1 -ethoxyethane 1 -ethoxypropane

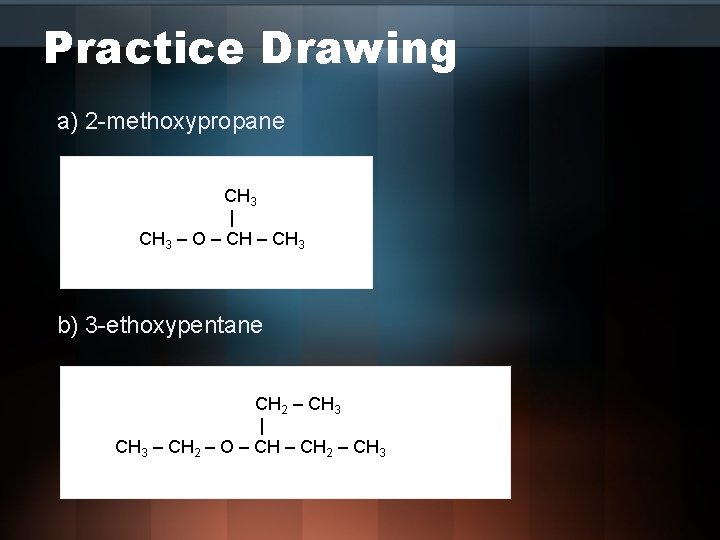
Practice Drawing a) 2 -methoxypropane CH 3 | CH 3 – O – CH 3 b) 3 -ethoxypentane CH 2 – CH 3 | CH 3 – CH 2 – O – CH 2 – CH 3