ALCOHOL Rev 3 09 Characteristics of Alcohol Types
![ALCOHOL ] ] Rev. 3. 09 Characteristics of Alcohol Types of Alcohol Ethanol Production ALCOHOL ] ] Rev. 3. 09 Characteristics of Alcohol Types of Alcohol Ethanol Production](https://slidetodoc.com/presentation_image_h2/568d4b0e7ad845505a187aab2dafaac9/image-1.jpg)
![CHARACTERISTICS OF ALCOHOL ] Aliphatic: Compounds composed of carbon and hydrogen atoms. ] Hydroxyl CHARACTERISTICS OF ALCOHOL ] Aliphatic: Compounds composed of carbon and hydrogen atoms. ] Hydroxyl](https://slidetodoc.com/presentation_image_h2/568d4b0e7ad845505a187aab2dafaac9/image-2.jpg)







- Slides: 16
![ALCOHOL Rev 3 09 Characteristics of Alcohol Types of Alcohol Ethanol Production ALCOHOL ] ] Rev. 3. 09 Characteristics of Alcohol Types of Alcohol Ethanol Production](https://slidetodoc.com/presentation_image_h2/568d4b0e7ad845505a187aab2dafaac9/image-1.jpg)
ALCOHOL ] ] Rev. 3. 09 Characteristics of Alcohol Types of Alcohol Ethanol Production 3 -1
![CHARACTERISTICS OF ALCOHOL Aliphatic Compounds composed of carbon and hydrogen atoms Hydroxyl CHARACTERISTICS OF ALCOHOL ] Aliphatic: Compounds composed of carbon and hydrogen atoms. ] Hydroxyl](https://slidetodoc.com/presentation_image_h2/568d4b0e7ad845505a187aab2dafaac9/image-2.jpg)
CHARACTERISTICS OF ALCOHOL ] Aliphatic: Compounds composed of carbon and hydrogen atoms. ] Hydroxyl groups: molecules consisting of an oxygen atom and a hydrogen atom. ] Hydrophilic: substances that can enter into a charged interaction with water molecules. ] Tasteless ] Poisonous Rev. 3. 09 3 -2

TYPES OF ALCOHOL METHANOL: Simplest form of alcohol. CH OH Commonly referred to as wood alcohol Common uses include solvents, Paint remover, and fuel. Rev. 3. 09 Lethal dose 4 fluid ounces ( 100 – 125 ml) 3 -3

TYPES OF ALCOHOL ISOPROPANOL: C 3 H 8 O Commonly referred to as rubbing alcohol. Common uses include disinfectant and common solvents. Twice as toxic as ethanol. Rev. 3. 09 3 -4

TYPES OF ALCOHOL ETHYLENE GLYCOL: C 2 H 4(OH)2 Common use as automotive antifreeze. Lethal dose 1 fluid ounce 30 ml). Rev. 3. 09 3 -5

TYPES OF ALCOHOL ETHANOL: C 2 H 5 OH Commonly referred to as grain alcohol or drinking alcohol. Commonly used as fuel additive, alcoholic beverages, and antiseptics. Lethal dose 4. 5 fluid ounces ( 130 ml). Rev. 3. 09 3 -6

ETHANOL Ethanol Production Produced by the process of natural fermentation Sugar + Yeast = Alcohol and Gas CO 2 Malting process used for grains Process of converting starches to sugar Allow the grain to sprout and add an enzyme (beta amylase) to break down starch and release the sugar Resulting mixture is called MASH Rev. 3. 09 3 -7

ETHANOL Ethanol Production Fermentation Begin with a sugar substance fruit, grapes, grains, Carbon, Hydrogen, Oxygen Add yeast Results Alcohol: C 2 H 5 OH Gas: Rev. 3. 09 CO 2 3 -8

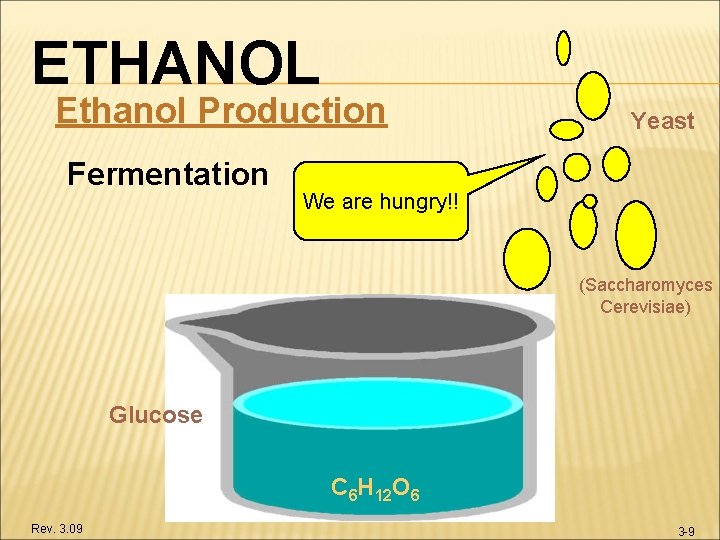
ETHANOL Ethanol Production Fermentation Yeast We are hungry!! (Saccharomyces Cerevisiae) Glucose C 6 H 12 O 6 Rev. 3. 09 3 -9

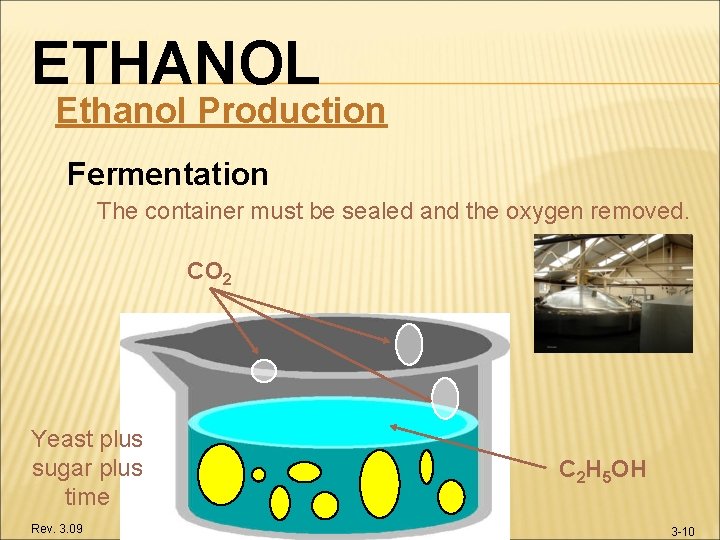
ETHANOL Ethanol Production Fermentation The container must be sealed and the oxygen removed. CO 2 Yeast plus sugar plus time Rev. 3. 09 C 2 H 5 OH 3 -10

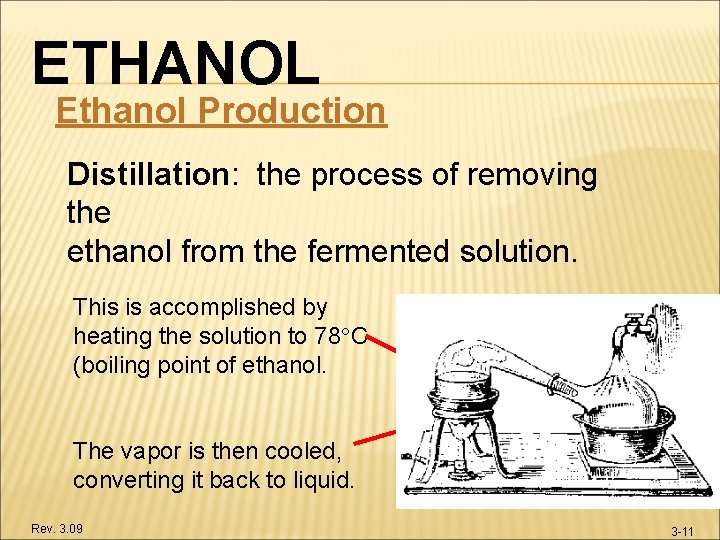
ETHANOL Ethanol Production Distillation: the process of removing the ethanol from the fermented solution. This is accomplished by heating the solution to 78 C (boiling point of ethanol. The vapor is then cooled, converting it back to liquid. Rev. 3. 09 3 -11

ETHANOL Ethanol Production Congeners: are added for aroma and flavoring of alcoholic beverages. They DO NOT contribute to intoxication. Rev. 3. 09 3 -12

ETHANOL Ethanol Production Proof: Rev. 3. 09 The term originated in the 18 th century, when payments to British sailors included rations of rum. To ensure that it had not been watered down and was of good quality, it was "proved" by dousing gunpowder in the liquor, and testing to see if it would ignite. If it did not, the solution contained too much water—and the alcohol content was considered low or "underproof". 3 -13

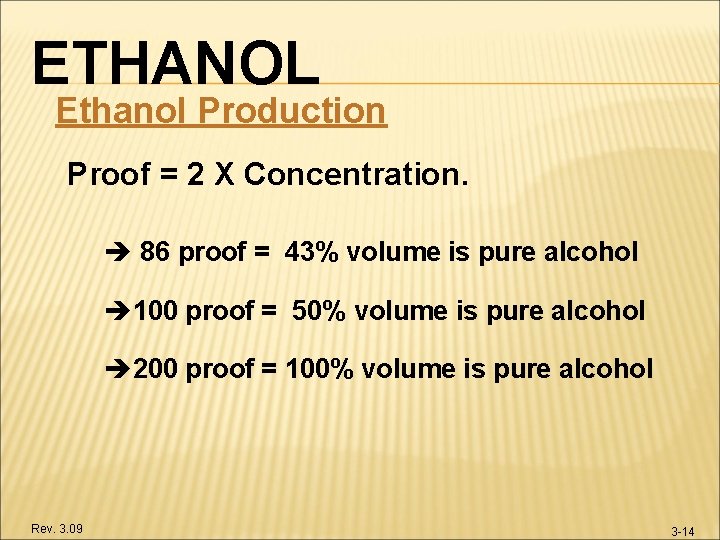
ETHANOL Ethanol Production Proof = 2 X Concentration. 86 proof = 43% volume is pure alcohol 100 proof = 50% volume is pure alcohol 200 proof = 100% volume is pure alcohol Rev. 3. 09 3 -14

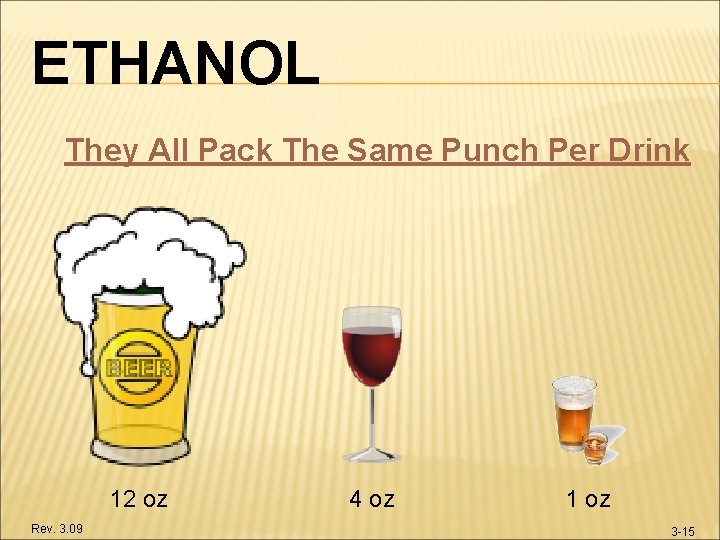
ETHANOL They All Pack The Same Punch Per Drink 12 oz Rev. 3. 09 4 oz 1 oz 3 -15

Questions Rev. 3. 09 3 -16