Alcohol Aldehydes and Acids Some organic Functional Groups



- Slides: 13

Alcohol, Aldehydes and Acids Some organic Functional Groups

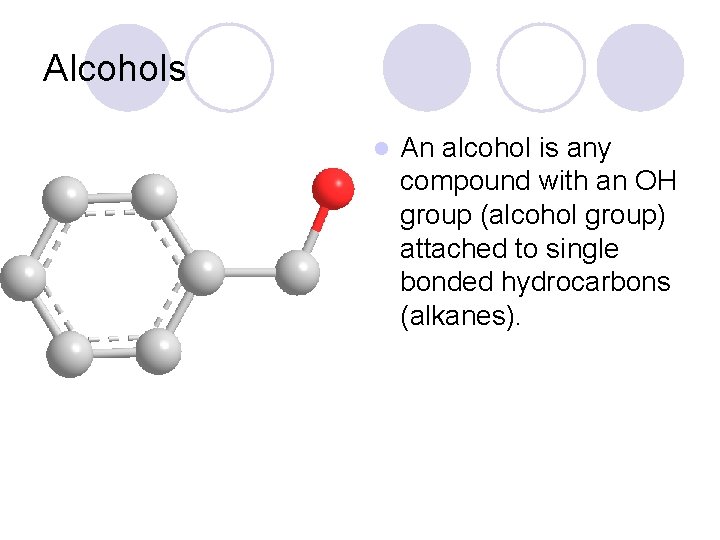
Alcohols l An alcohol is any compound with an OH group (alcohol group) attached to single bonded hydrocarbons (alkanes).

Ethanol l Ethanol, when fermented from sugar, is the alcohol in beverages. It can also be made from ethene by the addition of water for nonbeverage use, like an additive to gassoline to make "gasahol. " 2 -Propanol (better known as isopropyl alcohol) is in (with some water) rubbing alcohol. It is also used in gasoline to prevent freezing of the gas line in automobiles by keeping excess moisture dissolved in the gasoline.


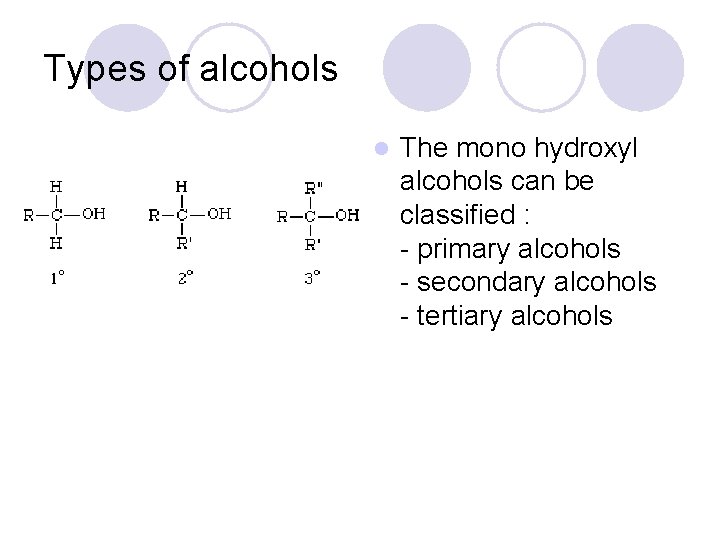
Types of alcohols l The mono hydroxyl alcohols can be classified : - primary alcohols - secondary alcohols - tertiary alcohols

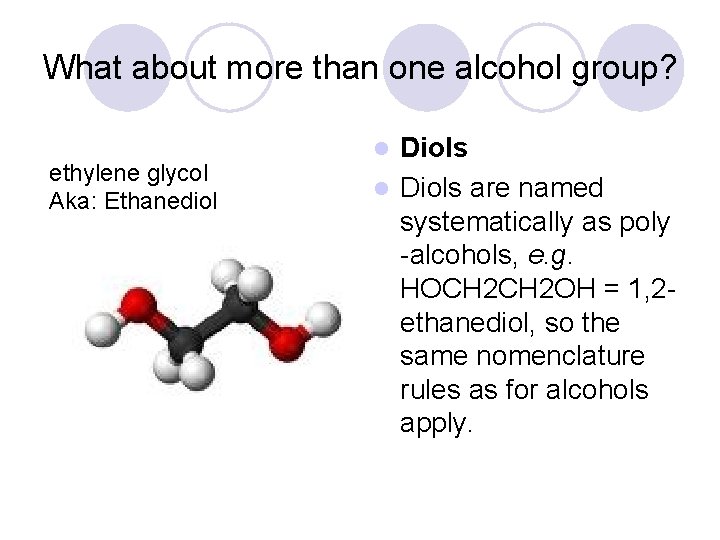
What about more than one alcohol group? ethylene glycol Aka: Ethanediol Diols are named systematically as poly -alcohols, e. g. HOCH 2 OH = 1, 2 ethanediol, so the same nomenclature rules as for alcohols apply. l

Diols Nomenclature: Diols are named systematically as poly -alcohols, l e. g. HOCH 2 OH = 1, 2 -ethanediol, so the same nomenclature rules as for alcohols apply. l ethanediol l 1, 2 -propanediol l 1, 3 -propanediol l cis-1, 2 -cyclohexanediol l

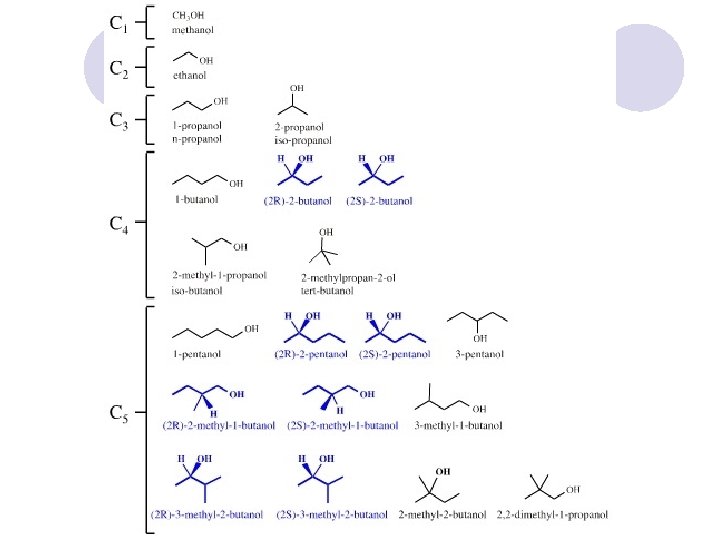
Alcohols l l l Alcohols (R-OH) take the suffix "-ol" with an infix numerical bonding position: CH 3 CH 2 OH is propan-1 -ol. The suffixes -diol, -triol, -tetraol, etc. , are used for multiple -OH groups: Ethylene glycol CH 2 OH is ethane-1, 2 -diol. If higher precedence functional groups are present (see order of precedence, below), the prefix "hydroxy" is used with the bonding position: CH 3 CHOHCOOH is 2 hydroxypropanoic acid.

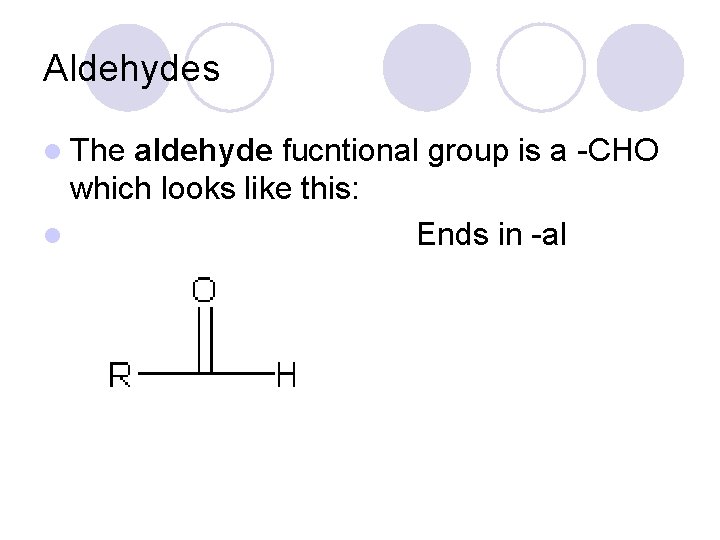
Aldehydes l The aldehyde fucntional group is a -CHO which looks like this: l Ends in -al

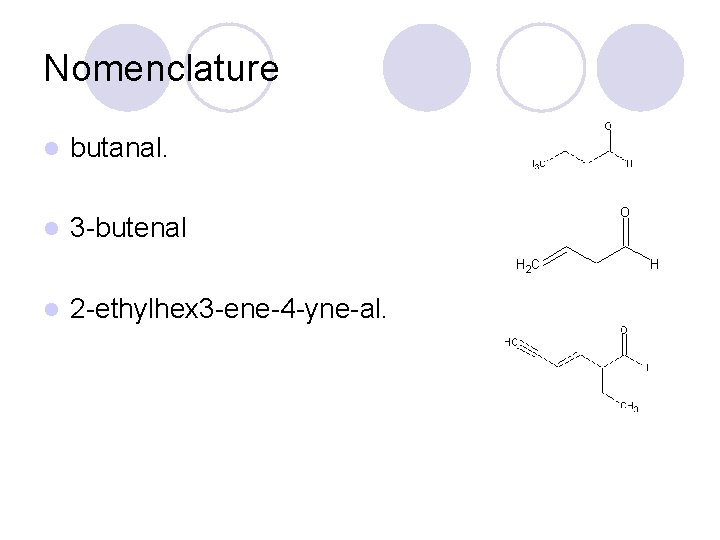
Nomenclature l butanal. l 3 -butenal l 2 -ethylhex 3 -ene-4 -yne-al.

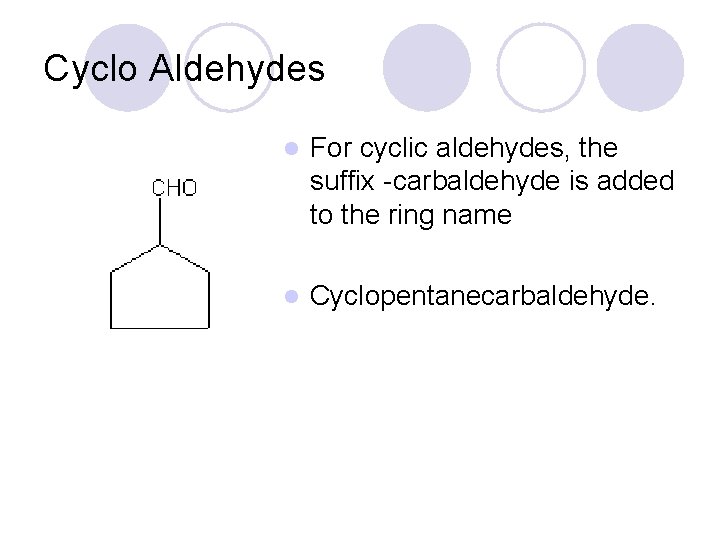
Cyclo Aldehydes l For cyclic aldehydes, the suffix -carbaldehyde is added to the ring name l Cyclopentanecarbaldehyde.

Carboxylic Acids l The naming of carboxylic acids is fairly simple. You simply find the longest carbon chain which includes the carboxylic group. Use that as the stem for the name, cross off the -e on the ending of the alkane name and replace it with -oic acid

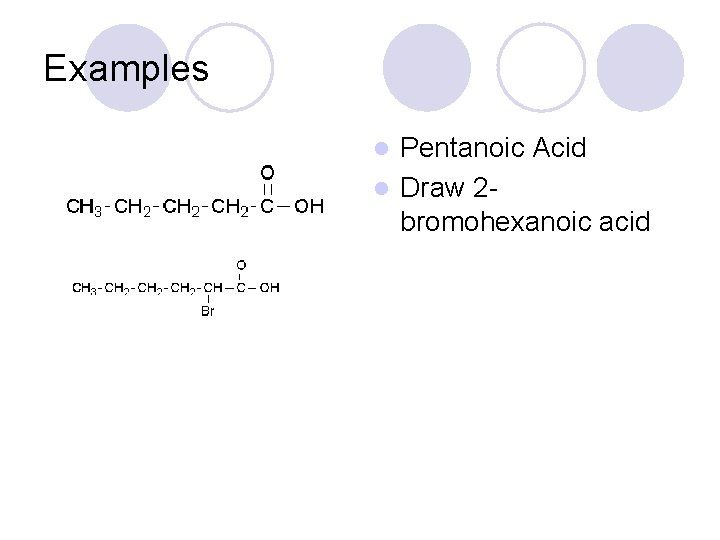
Examples Pentanoic Acid l Draw 2 bromohexanoic acid l