Airflow and Work of Breathing 2 High lung

Airflow and Work of Breathing 2. High lung compliance means the lungs and chest wall expand easily. – Compliance is decreased by a broken rib, or by diseases such as pneumonia or emphysema.

Airflow and Work of Breathing
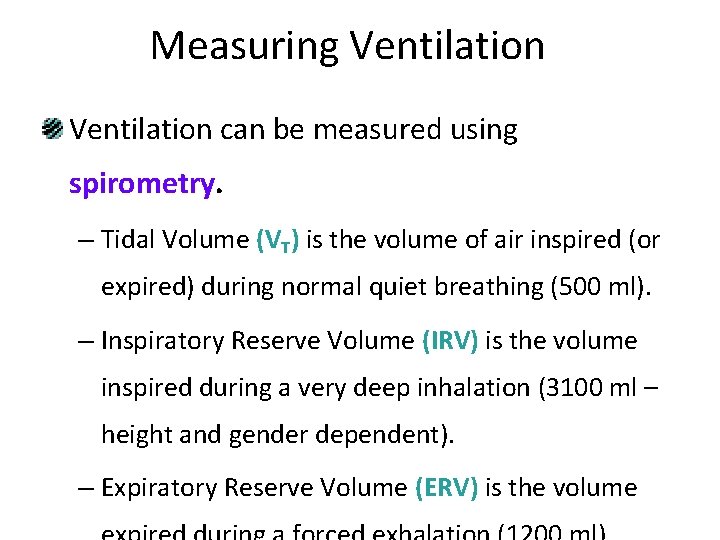
Measuring Ventilation can be measured using spirometry. – Tidal Volume (VT) is the volume of air inspired (or expired) during normal quiet breathing (500 ml). – Inspiratory Reserve Volume (IRV) is the volume inspired during a very deep inhalation (3100 ml – height and gender dependent). – Expiratory Reserve Volume (ERV) is the volume
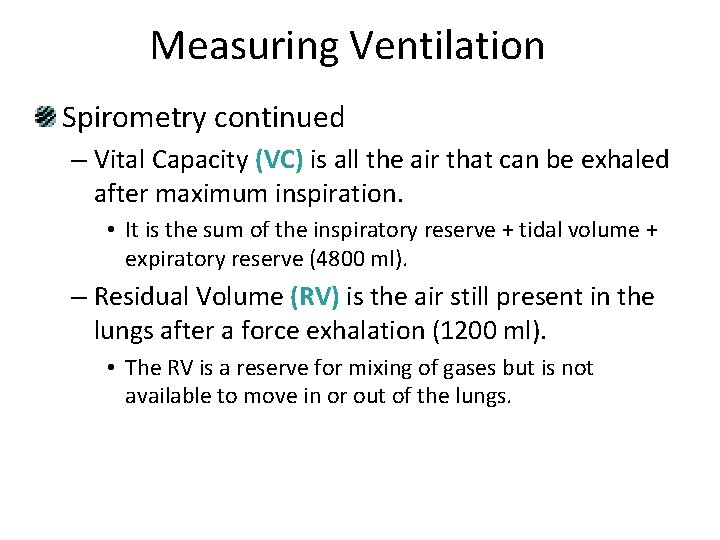
Measuring Ventilation Spirometry continued – Vital Capacity (VC) is all the air that can be exhaled after maximum inspiration. • It is the sum of the inspiratory reserve + tidal volume + expiratory reserve (4800 ml). – Residual Volume (RV) is the air still present in the lungs after a force exhalation (1200 ml). • The RV is a reserve for mixing of gases but is not available to move in or out of the lungs.

Measuring Ventilation Old and new spirometers used to measure ventilation.

Measuring Ventilation A graph of spirometer volumes and capacities
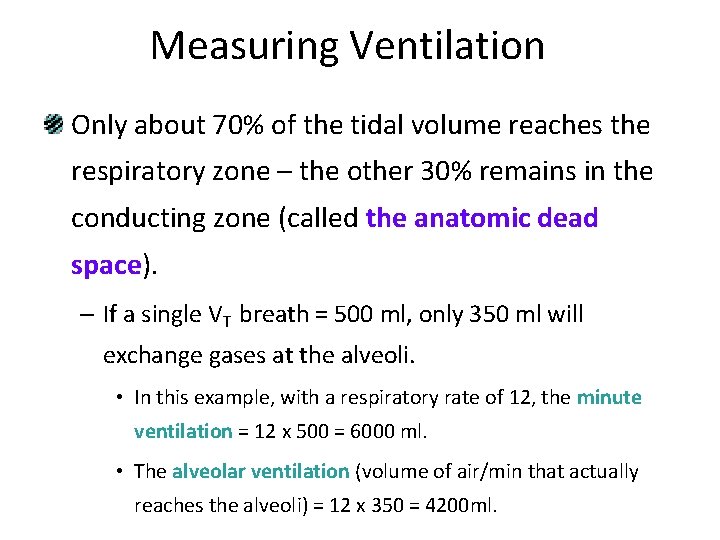
Measuring Ventilation Only about 70% of the tidal volume reaches the respiratory zone – the other 30% remains in the conducting zone (called the anatomic dead space). – If a single VT breath = 500 ml, only 350 ml will exchange gases at the alveoli. • In this example, with a respiratory rate of 12, the minute ventilation = 12 x 500 = 6000 ml. • The alveolar ventilation (volume of air/min that actually reaches the alveoli) = 12 x 350 = 4200 ml.
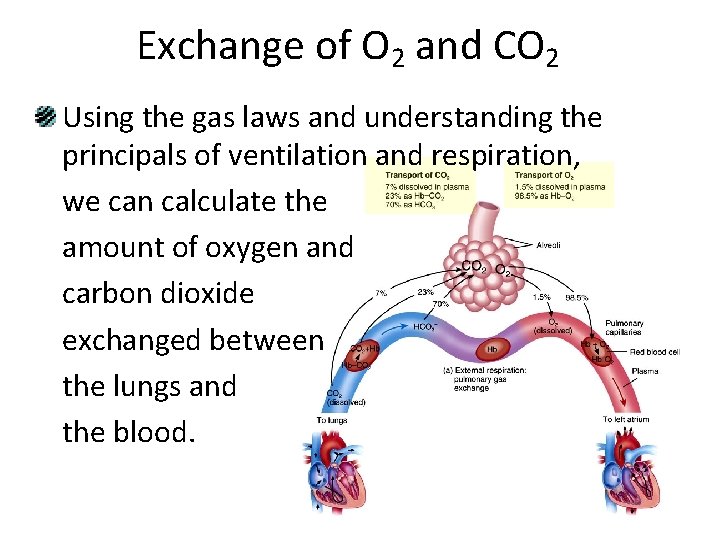
Exchange of O 2 and CO 2 Using the gas laws and understanding the principals of ventilation and respiration, we can calculate the amount of oxygen and carbon dioxide exchanged between the lungs and the blood.

Exchange of O 2 and CO 2 • Dalton’s Law states that each gas in a mixture of gases exerts its own pressure as if no other gases were present. – The pressure of a specific gas is the partial pressure Pp. – Total pressure is the sum of all the partial pressures. – Atmospheric pressure (760 mm. Hg) = PN 2 + PO 2 + PH 2 O + PCO 2 + Pother gases • Since O 2 is 21% of the atmosphere, the PO 2 is 760 x 0. 21 = 159. 6 mm. Hg.
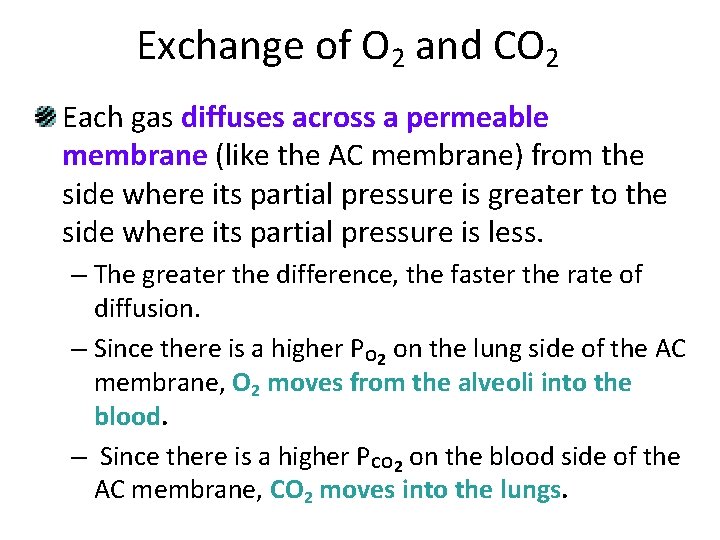
Exchange of O 2 and CO 2 Each gas diffuses across a permeable membrane (like the AC membrane) from the side where its partial pressure is greater to the side where its partial pressure is less. – The greater the difference, the faster the rate of diffusion. – Since there is a higher PO 2 on the lung side of the AC membrane, O 2 moves from the alveoli into the blood. – Since there is a higher PCO 2 on the blood side of the AC membrane, CO 2 moves into the lungs.
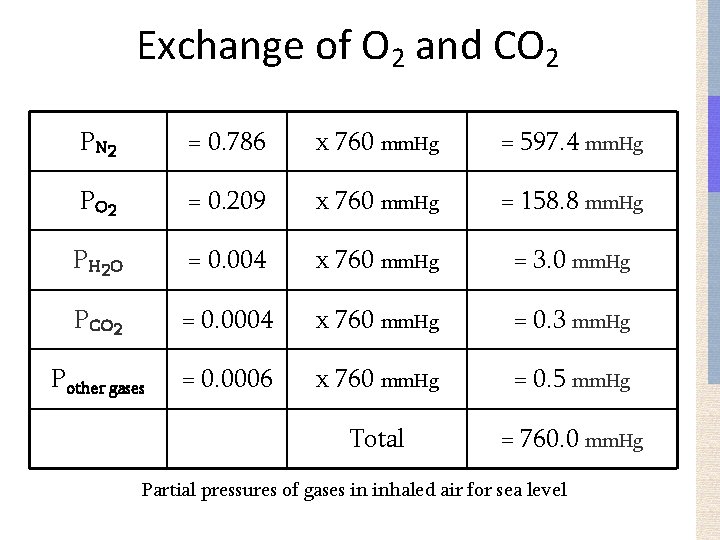
Exchange of O 2 and CO 2 P N 2 = 0. 786 x 760 mm. Hg = 597. 4 mm. Hg P O 2 = 0. 209 x 760 mm. Hg = 158. 8 mm. Hg P H 2 O = 0. 004 x 760 mm. Hg = 3. 0 mm. Hg PCO 2 = 0. 0004 x 760 mm. Hg = 0. 3 mm. Hg Pother gases = 0. 0006 x 760 mm. Hg = 0. 5 mm. Hg Total = 760. 0 mm. Hg Partial pressures of gases in inhaled air for sea level
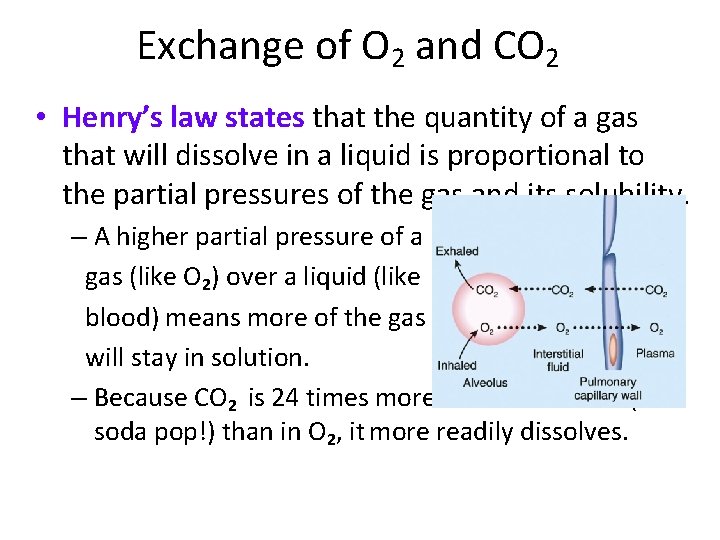
Exchange of O 2 and CO 2 • Henry’s law states that the quantity of a gas that will dissolve in a liquid is proportional to the partial pressures of the gas and its solubility. – A higher partial pressure of a gas (like O 2) over a liquid (like blood) means more of the gas will stay in solution. – Because CO 2 is 24 times more soluble in blood (and soda pop!) than in O 2, it more readily dissolves.
Exchange of O 2 and CO 2 Even though the air we breathe is mostly N 2, very little dissolves in blood due to its low solubility. – Decompression sickness (“the bends”) is a result of the comparatively insoluble N 2 being forced to dissolve into the blood and tissues because of the very high pressures associated with diving. • By ascending too rapidly, the N 2 rushes out of the tissues and the blood so forcefully as to cause vessels to “pop” and cells to die.
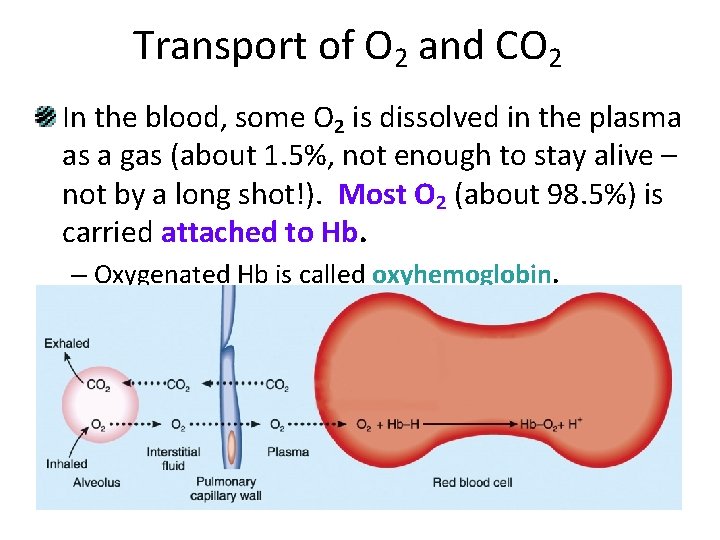
Transport of O 2 and CO 2 In the blood, some O 2 is dissolved in the plasma as a gas (about 1. 5%, not enough to stay alive – not by a long shot!). Most O 2 (about 98. 5%) is carried attached to Hb. – Oxygenated Hb is called oxyhemoglobin.
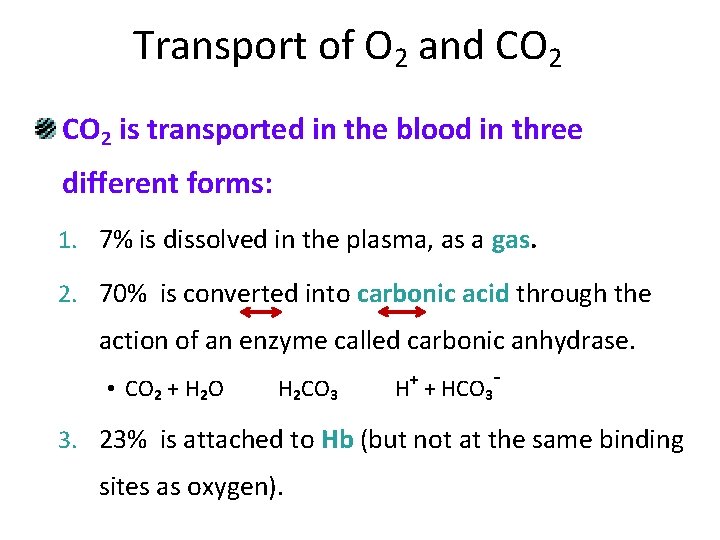
Transport of O 2 and CO 2 is transported in the blood in three different forms: 1. 7% is dissolved in the plasma, as a gas. 2. 70% is converted into carbonic acid through the action of an enzyme called carbonic anhydrase. + • CO 2 + H 2 O H 2 CO 3 H + HCO 3 - 3. 23% is attached to Hb (but not at the same binding sites as oxygen).
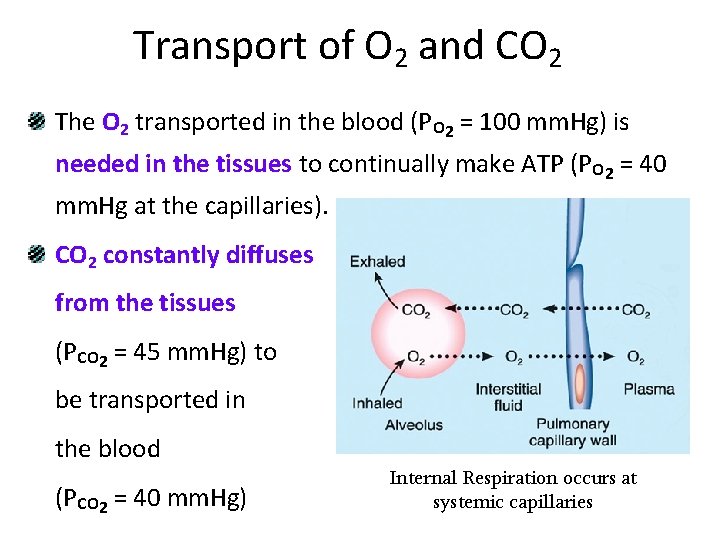
Transport of O 2 and CO 2 The O 2 transported in the blood (PO 2 = 100 mm. Hg) is needed in the tissues to continually make ATP (PO 2 = 40 mm. Hg at the capillaries). CO 2 constantly diffuses from the tissues (PCO 2 = 45 mm. Hg) to be transported in the blood (PCO 2 = 40 mm. Hg) Internal Respiration occurs at systemic capillaries
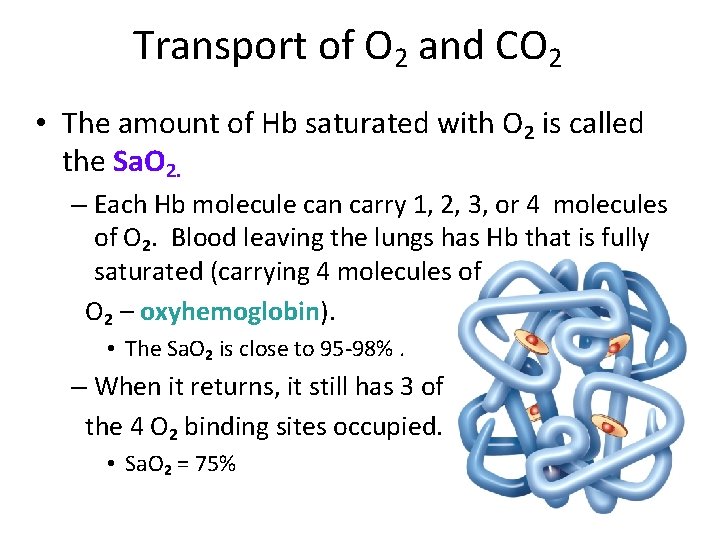
Transport of O 2 and CO 2 • The amount of Hb saturated with O 2 is called the Sa. O 2. – Each Hb molecule can carry 1, 2, 3, or 4 molecules of O 2. Blood leaving the lungs has Hb that is fully saturated (carrying 4 molecules of O 2 – oxyhemoglobin). • The Sa. O 2 is close to 95 -98%. – When it returns, it still has 3 of the 4 O 2 binding sites occupied. • Sa. O 2 = 75%
- Slides: 17