AIR POLLUTION Sources concentration and measurement Dr Mai









- Slides: 17

AIR POLLUTION Sources , concentration and measurement Dr : Mai El gammal Presented by : Somaya Hany Abo Samra

SPECIFIC AIR POLLUTANTS (1) Sulfur dioxide (2) Suspended particulate matter (3) Oxides of nitrogen (4) Carbon monoxide (5) Hydrocarbons (6) Secondary pollutants (ozone & peroxyacetyl nitrate )

SULFUR DIOXIDE • Sources : • Major source is combustion of fossil fuels that contain sulfur • ( coal & fuel oil ) have high sulfur content • (natural gas , petrol & diesel fuels ) have low sulfur content • In UK combustion of coal in power stations is the most major source of SO 2 • Effect of sulfur dioxide : • Atmospheric SO 2 is oxidized forming sulfuric acid that may be incorporated into rain or dry deposited as fine droplets causes acidification of soil and surface waters that called ( acid rain ) as in Europe

• Measurement of sulfur dioxide : • 1 Absorption of sulfur dioxide (SO 2) in hydrogen peroxide solution (H 2 O 2) to form sulfuric acid ( H 2 SO 4), then the resultant acid has been determined by acid – base titration to interference by other gaseous , acidic or base compounds such as nitric acid or ammonia • 2 Measure sulfur dioxide (SO 2) by measurement of fluorescence excited by radiation in the region (commercial instrument) called sulfate analysis gas phase fluorescence

SUSPENDED PARTICULATE MATTER • Including both organic and inorganic substances with diameters ranging from less than 10 nm to greater than 100 µm • Sources: • Transient nuclei : formed by condensation of hot vapors or gas to particle conversion process • Accumulation range : formed by rain water or dry deposit of primary particles from motor vehicle exhaust and sulfuric acid or, coagulate with other fine particles • Coarse particles : growth through condensation of low volatility materials or mechanical generation such as wind blown dust , sea spray and volcanic particles

• Measurement of suspended particles: • 1) Black smoke : related to blackness or soiling capacity of particles • 2) Measuring of suspended particles : depend upon gravimetric determination of particle mass • 3) Total suspended particulate matter(TSP): used to describe the fraction of particles collected on a filter using the US high volume sampler

• Black smoke: • In UK inventories are generated by measuring the mass emission rates of particles from the various sources and then multiplying by a blackness index which describes the relative darkness of the different smokes • Coal smoke : index of 1 * diesel smoke : index of 3 • Main source of black smoke : • Road transport , diesel fuel component and coal burning in domestic premises • Region : London 94% black smoke from road traffic • Measurement: in UK air is drawn through a cellulose filter upon which particles are collected. At the end of sampling period the ability of the filter surface to reflect light is determined quantitatively and reflectance is related to concentration of standard smoke using a calibration graph (dominated by coal smoke )

• Gravimetrically determined particulate matter: • ( include primary and secondary particles) • Sources : • Dominant sources: road traffic emissions , ammonium sulfate , ammonium nitrate particles and other sources as sea spray and wind blown dust contribute to urban PM 10 • Minor sources : particles from building work , biological particles such as pollens spores and bacteria • Measurement of PM 10 : • 1) using of high volume air sampler capable of drawing air through a filter ae a rate of about 1 m³ min‾¹ through 10 µm size selective inlet • 2) using the Tapered Element Oscillating Microbalance(TEOM)

• EFFECT OF AIRBORNE PARTICLES : • Soiling of building • Loss of visibility • Scatter and absorb light cause deterioration in the quality of image transmission through the atmosphere • Formation of smog caused by low level emissions from coal combustion , during periods of meteorology smoke + fog ) led to losses in visibility (smog=

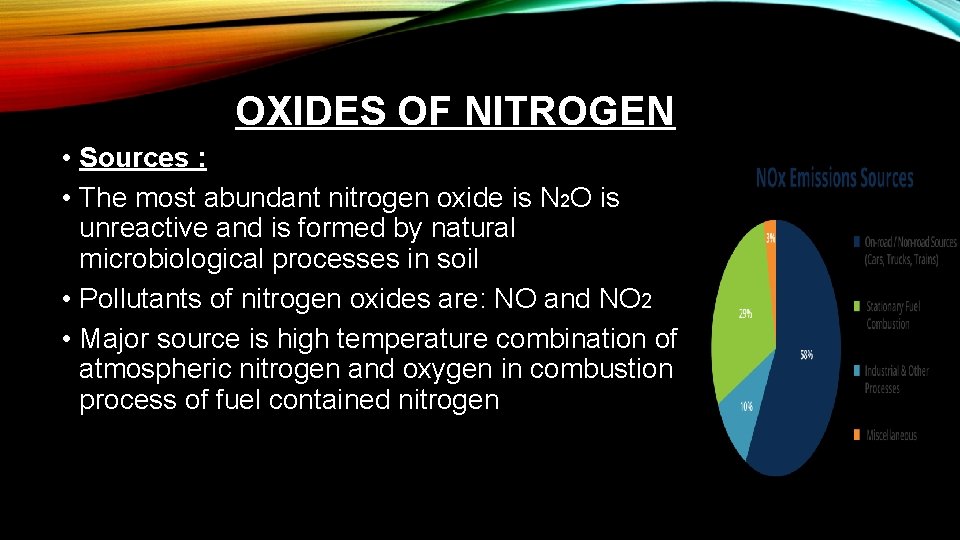
OXIDES OF NITROGEN • Sources : • The most abundant nitrogen oxide is N 2 O is unreactive and is formed by natural microbiological processes in soil • Pollutants of nitrogen oxides are: NO and NO 2 • Major source is high temperature combination of atmospheric nitrogen and oxygen in combustion process of fuel contained nitrogen

• Effect of nitrogen oxides: • Direct effect : • Human respiratory tract and irritation • Damage of plant • Indirect effect : • Arise from the central role of NO 2 in photochemical smog reacting • Its oxidation to nitric acid contributing to acid rain problems

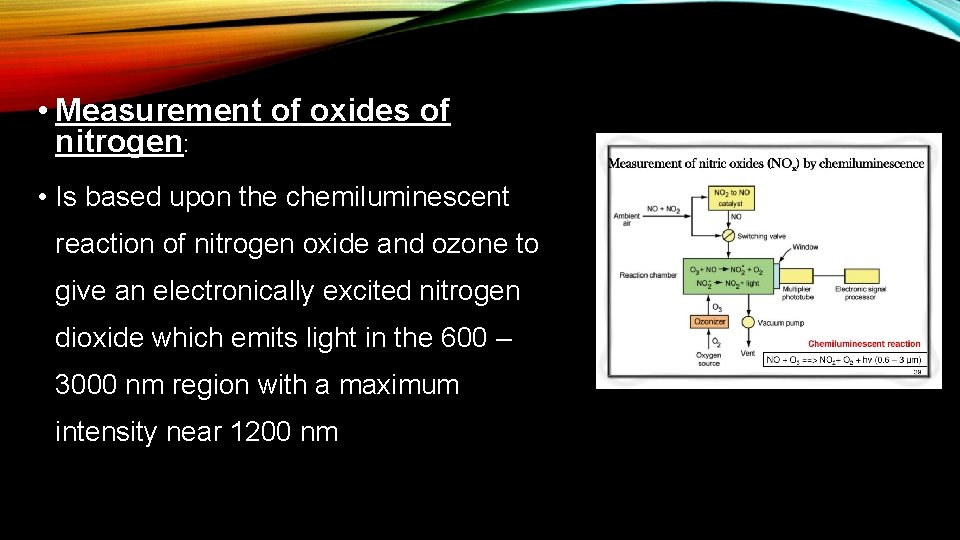
• Measurement of oxides of nitrogen: • Is based upon the chemiluminescent reaction of nitrogen oxide and ozone to give an electronically excited nitrogen dioxide which emits light in the 600 – 3000 nm region with a maximum intensity near 1200 nm

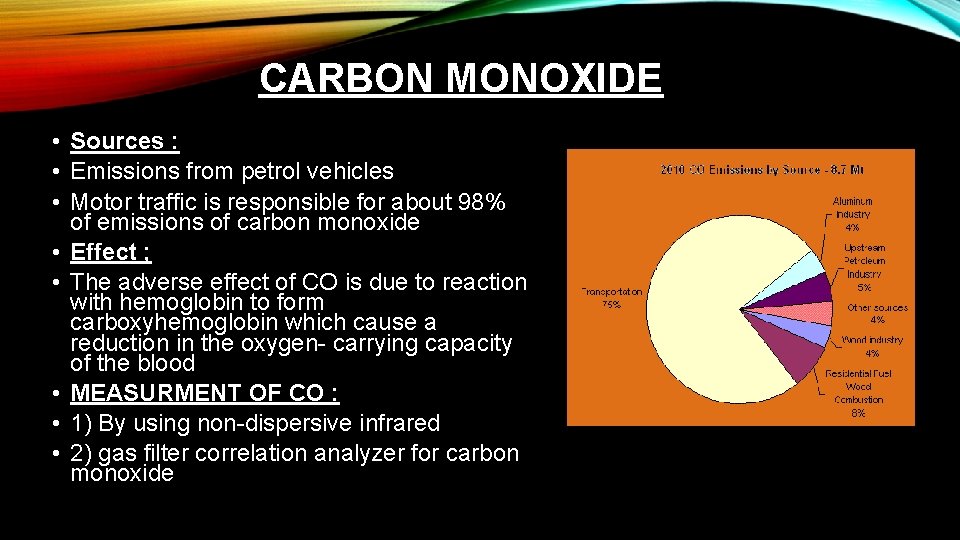
CARBON MONOXIDE • Sources : • Emissions from petrol vehicles • Motor traffic is responsible for about 98% of emissions of carbon monoxide • Effect ; • The adverse effect of CO is due to reaction with hemoglobin to form carboxyhemoglobin which cause a reduction in the oxygen- carrying capacity of the blood • MEASURMENT OF CO : • 1) By using non-dispersive infrared • 2) gas filter correlation analyzer for carbon monoxide

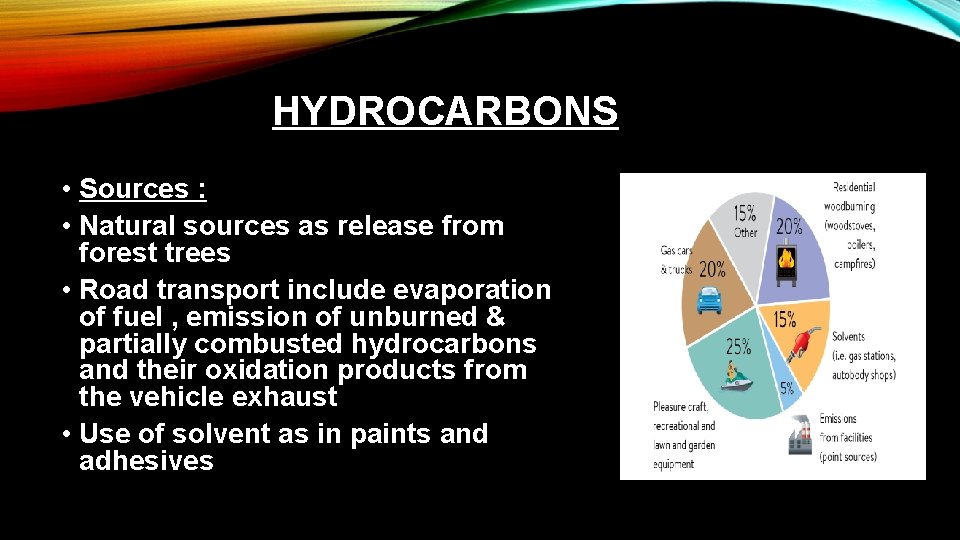
HYDROCARBONS • Sources : • Natural sources as release from forest trees • Road transport include evaporation of fuel , emission of unburned & partially combusted hydrocarbons and their oxidation products from the vehicle exhaust • Use of solvent as in paints and adhesives

• Effect of hydrocarbons: • 1) direct toxicity of some compounds particularly benzene and 1, 3 –butadiene are chemically carcinogens • 2) their role as precursors of photochemical ozone • Measurement of hydrocarbons: • Require a pre-concentration stage in which air is drawn through an absorbent as porous polymer or activated carbon where freeze - out of the compounds by reduced temperature occurs followed by injection into gas chromatograph • Detection may be by flame ionization or mass spectrometer • The UK Hydrocarbon network

SECONDARY POLLUTANTS • 1) Ozone • Sources: • Severe photochemical smog as in southern California • From reaction of hydrocarbons and NOX emissions in the presence of sunlight as in Europe • Effect : • Effect on human lung function • Damage to crop plants • Measurement: • The UV absorption of ozone at 254 nm may be used for its determination at level down to 1 ppb

SECONDARY POLLUTANTS • Peroxyacetyl nitrate (PAN) • Sources: • Is a product of atmospheric photo chemical reactions and is a characteristic of photochemical smog • Chemical analysis of PAN: • Determined by long- path IR measurements • Lower concentration may be measured by preconcentration of PAN from the air