Air Pollution n 1 What is air pollution




























- Slides: 41

Air Pollution n 1. What is air pollution? n 2. Sources of air pollutants n 3. Air pollutants n 4. Ozone and CFC n 5. Photo chemical smog n 6. Acid rain n 7. Ways to reduce air pollution

What is air pollution? ? - the presence of undesirable substances in air at a level that is high enough to cause damage to our health or properties

Sources of air pollutants human activities §burning of fossil fuel § use of motor vehicles §electric power station §factories §incinerators

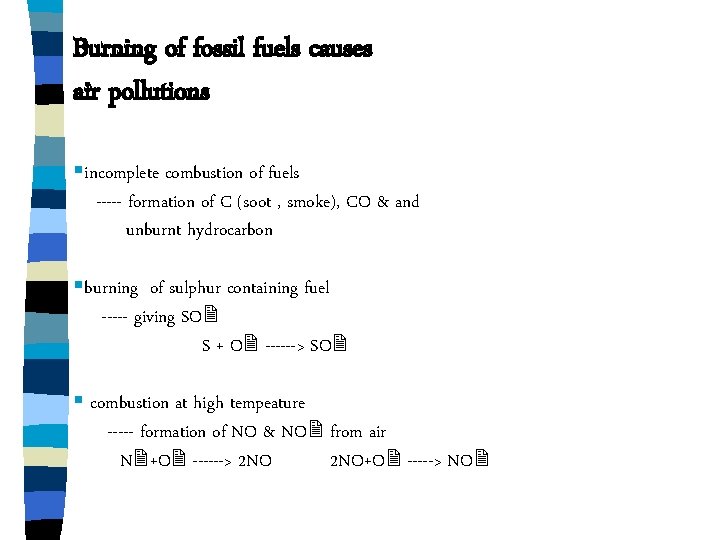
Burning of fossil fuels causes air pollutions §incomplete combustion of fuels ----- formation of C (soot , smoke), CO & and unburnt hydrocarbon §burning of sulphur containing fuel ----- giving SO S + O ------> SO § combustion at high tempeature ----- formation of NO & NO from air N +O ------> 2 NO+O -----> NO

Äcombustion of leaded petrol ----- forming lead and Pb. O Ä burning of coal with some metal compounds ----- giving ash e. g. soot

Air pollutants n n n 1. Carbon monoxide 2. Nitrogen oxide 3. sulphur dioxide 4. lead compounds 5. unburned hydrocarbons 6. particulates

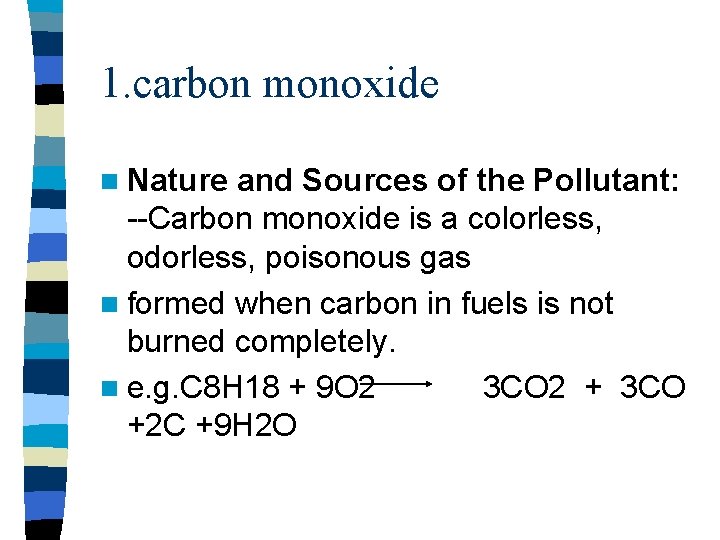
1. carbon monoxide n Nature and Sources of the Pollutant: --Carbon monoxide is a colorless, odorless, poisonous gas n formed when carbon in fuels is not burned completely. n e. g. C 8 H 18 + 9 O 2 3 CO 2 + 3 CO +2 C +9 H 2 O

Health and Environmental Effects: --Carbon monoxide enters the bloodstream and reduces oxygen delivery to the body's organs and tissues. -Low conc. : causes dizziness, headache, -High conc. : cause unconsciousness and even death

2. nitrogen oxides n Nature and Sources of the Pollutant: -Combustion of fuels in high temperature N 2 +O 2 NO 2 --Car exhaust, factories, power stations

Health and Environmental Effects: n Irritate and attack respiratory tracts and the lungs n Form acid rain

3. sulphur dioxide n Nature and Sources of the Pollutant: Burning of fuel which contain sulphur (impurities) -From factories, power station and incineration.

Health and Environmental effects: n Harmful respiratory system n Cause cancer and heart problems n Irritate the eyes n Causes acid rain

4. lead cpds. n Nature and Sources of the Pollutant: -Combustion of fuel of cars. -lead are added to increase the burning efficiency

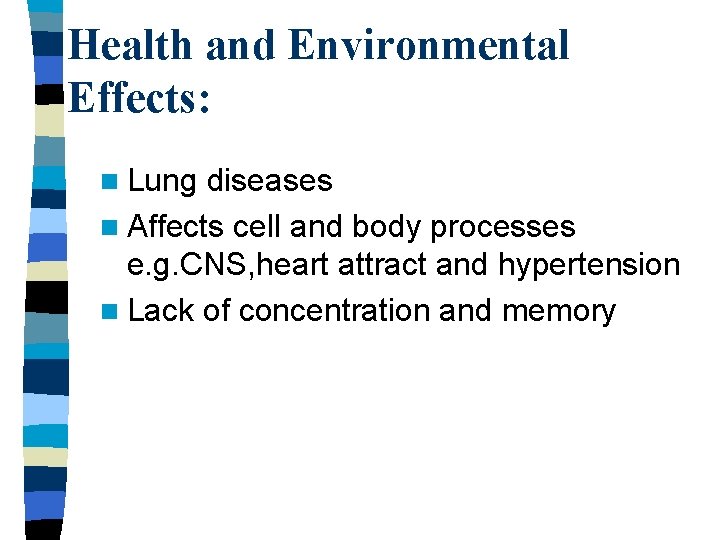
Health and Environmental Effects: n Lung diseases n Affects cell and body processes e. g. CNS, heart attract and hypertension n Lack of concentration and memory

5. unburned hydrocarbons n Nature and Sources of the Pollutant: -evaporation of petrol -exhaust of incompletely burnt of fuel(factories , power plants)

Health and Environmental Effects: n Causing cancer n Formation of photochemical smog

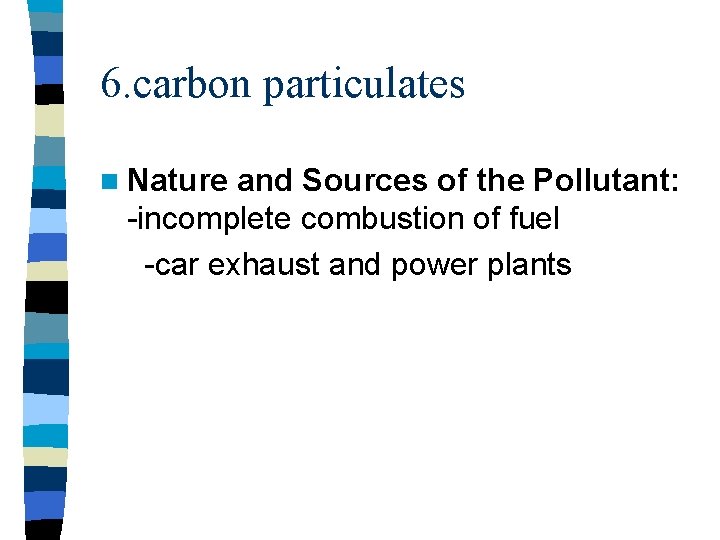
6. carbon particulates n Nature and Sources of the Pollutant: -incomplete combustion of fuel -car exhaust and power plants

Health and Environmental Effects: n Suspended particulates cause lung cancer and diseases such as tuberculosis

Ozone and CFC Formation of ozone n 1. Reaction between NO 2 and hydrocarbon n 2. From air by electrical sparks( in car engine) & in electric machine(photo copier) n 3. From atmospheric O 2 by absorption of UV radiation n O 2 O + O n O + O 2 O 3 n O 3 O 2 + O (dissociation of O 3) n

it is very important as the screening effect of ozone prevents the harmful UV rays from reaching the earth crust. n * 1 & 2 are formed in lower atm. , but 3 is in upper atm. n

n Properties of ozone n 1. pale blue gas n 2. pungent smell n 3. harmless at low concentration n 4. causes breathing problems & headache (if conc. >100 ppm) n Why do we need ozone? n It can filter out 99% if the dangerous UV radiation from the sun. Thus, stop skin cancer, eye contract and crop-yield reduction

n Properties of CFC n 1. chemically unreactive n 2. low flammability n 3. highly volatile n 4. low toxicity n Uses of CFC n 1. Aerosol propellants eg trichlorofluromethan(CCl 3 F), dichlorodifluromethane(CCl 2 F 2) n Since CFC is very volatile, it can produce high pressure to propel out eg insecticide

n 2. Solvents in cleaning agents for electronic component eg tricholorotrifluro ethane ( CCl 2 F-CCl. F 2 ) n CFC can dissolve grease & be removed by evaporation n 3. Refrigerants eg Freon( CCl 2 F 2) n It is highly volatile that it absorbs heat of vaporization during evaporation => cooling of surrounding

n 4. Blowing agents in foam plastic manufacturing eg CCl 3 F n CFC is incorporated into monomers. It is then vaporized by the heat evolved during polymerization. Tiny bubbles in plastic formed. n Why does CFC accumulate in stratosphere? n Since CFC is easily transported, Cl radicals are formed, CFC is insoluble in water, CFC accumulates.

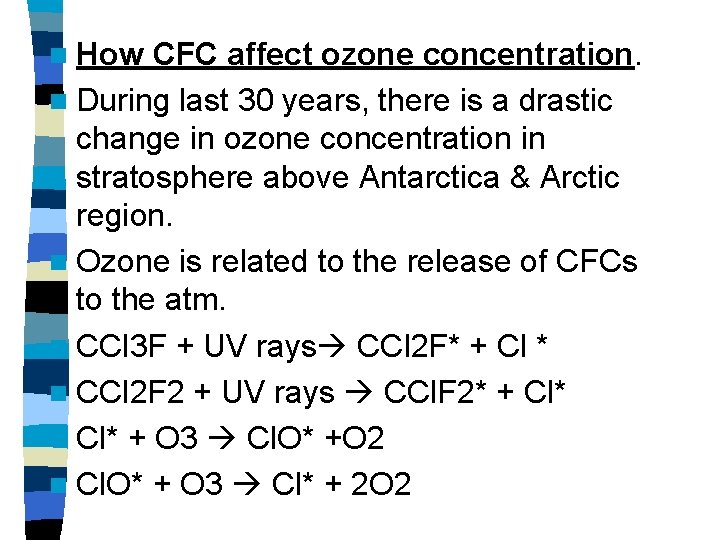
n How CFC affect ozone concentration. n During last 30 years, there is a drastic change in ozone concentration in stratosphere above Antarctica & Arctic region. n Ozone is related to the release of CFCs to the atm. n CCl 3 F + UV rays CCl 2 F* + Cl * n CCl 2 F 2 + UV rays CCl. F 2* + Cl* n Cl* + O 3 Cl. O* +O 2 n Cl. O* + O 3 Cl* + 2 O 2

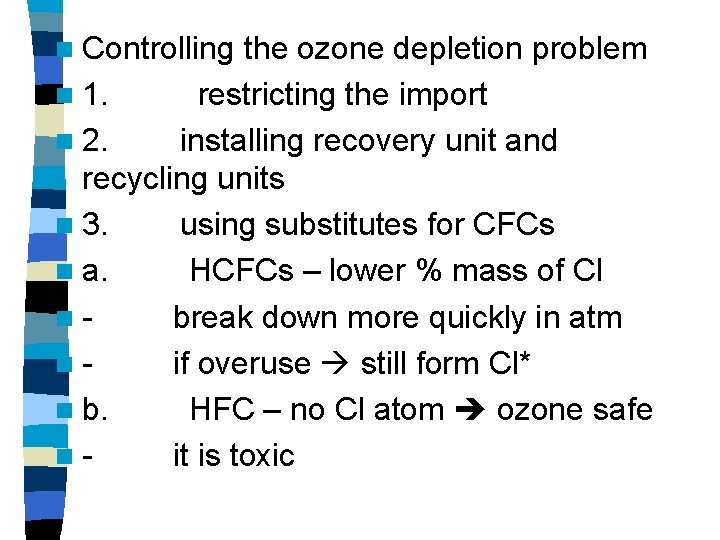
n Controlling the ozone depletion problem n 1. restricting the import n 2. installing recovery unit and recycling units n 3. using substitutes for CFCs n a. HCFCs – lower % mass of Cl nbreak down more quickly in atm nif overuse still form Cl* n b. HFC – no Cl atom ozone safe nit is toxic

n c. hydrocarbon (propane & butane) n - no Cl ozone safe n - cheap & readily available n - flmmable and poisonous can’t be widely used n d. water & steam n - effective in some cleaning application ( vaporizing grease at high temperature)

Photochemical Smog a mixture of pollutants including particulates, NO 2, peroxyacetyl nitrate( PAN), ozone, aldehydes, unreacted hydrocarbons , etc. Detecting photochemical smog §Brownish haze & painful eyes ---- brown colour is due to NO 2 ---- haze is due to particulates & droplets ---- pain felt is due to NO 2 , ozone & PAN

Factors leading to the formation of photochemical smog §reactants : unburnt hydrocarbons & NO 2 §initiating condition : UV rays §catalyst : particulates & other atmospheric pollutants §climatic factor : absence of strong winds will slow down the disappearance of smog §topographical factor : tall buildings will reduce the air flow in a crowded city

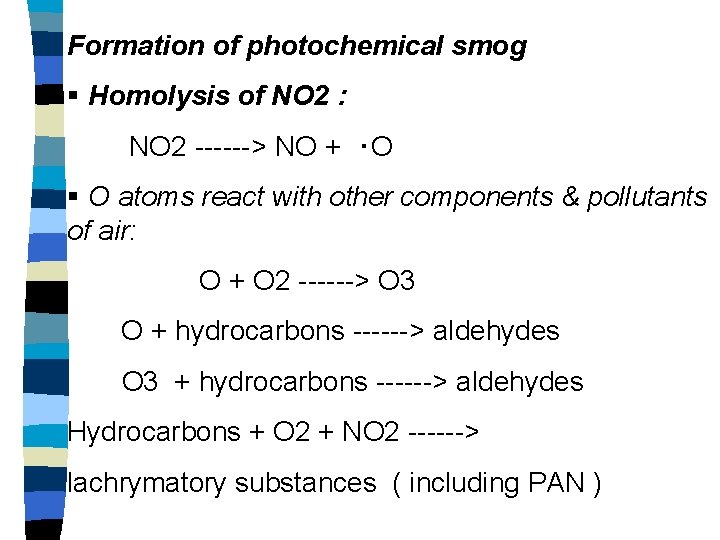
Formation of photochemical smog § Homolysis of NO 2 : NO 2 ------> NO + ‧O § O atoms react with other components & pollutants of air: O + O 2 ------> O 3 O + hydrocarbons ------> aldehydes O 3 + hydrocarbons ------> aldehydes Hydrocarbons + O 2 + NO 2 ------> lachrymatory substances ( including PAN )

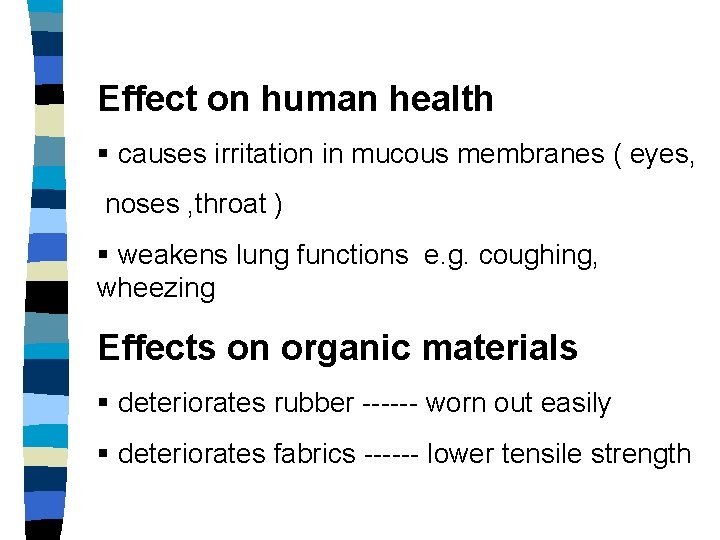
Effect on human health § causes irritation in mucous membranes ( eyes, noses , throat ) § weakens lung functions e. g. coughing, wheezing Effects on organic materials § deteriorates rubber ------ worn out easily § deteriorates fabrics ------ lower tensile strength

Acid Rain Unpolluted rain water is slightly acidic, with a p. H about 5. 7. 2. This is because rain water reacts with CO 2 in the air to form H 2 CO 3. 3. Rain water with p. H values lower than 5. 7 is called acid rain. 1.

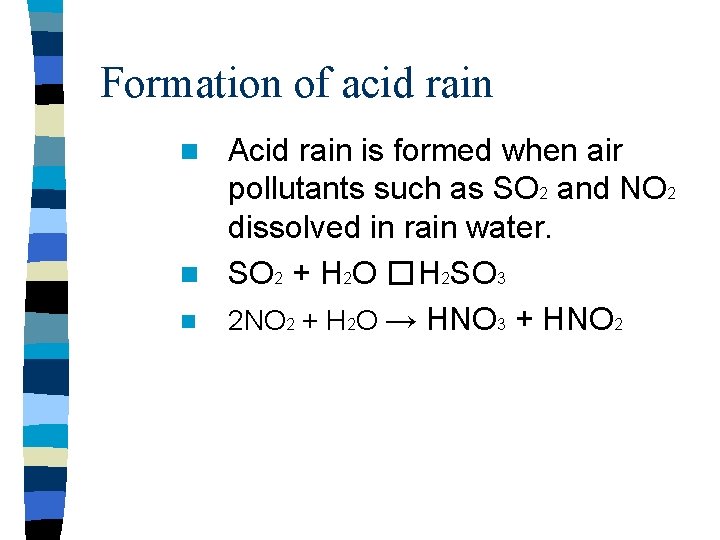
Formation of acid rain Acid rain is formed when air pollutants such as SO 2 and NO 2 dissolved in rain water. n SO 2 + H 2 O � H 2 SO 3 n 2 NO 2 + H 2 O → HNO 3 + HNO 2 n

Harmful effects of acid rain Soil become too acidic for plants to grow. Forest are seriously damaged. n River and lakes become more acidic. Some aquatic plants and animals may die. n The corrosion of metal will be speeded up. n Damages building materials e. g. limestone and marble. All these materials contain Ca. CO 3 which reacts with acid. n

Controlling of acid rain n Reducing pollution from motor vehicles and industries. n Neutralizing the acid s in soil and water using lime. n Burning less fossil fuels.

Methods of minimizing pollutants from the burning fuels n A. Motor vehicles 1. Fitting catalyst converters to motor vehicles. 2. Using unleaded petrol. -- lead emission into the air can be greatly reduced. 3. Using LPG instead of diesel. -- it is much cleaner fuel than diesel. 4. Using alcohol instead of petrol. -- it is much cleaner fuel than petrol.

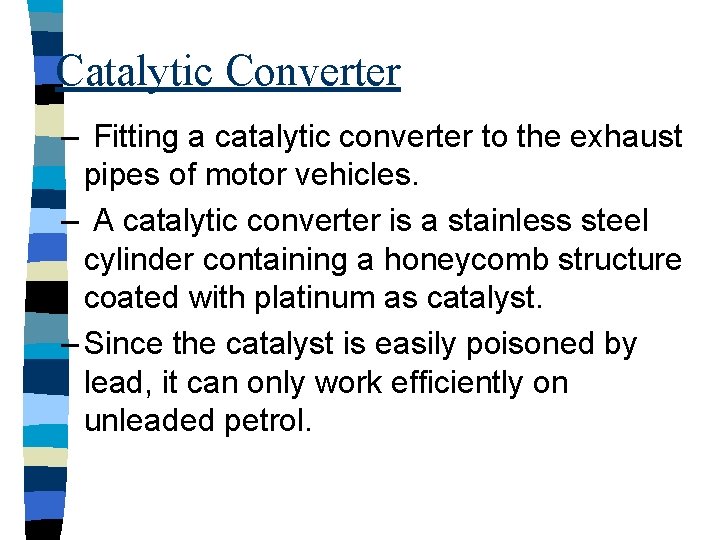
Catalytic Converter – Fitting a catalytic converter to the exhaust pipes of motor vehicles. – A catalytic converter is a stainless steel cylinder containing a honeycomb structure coated with platinum as catalyst. – Since the catalyst is easily poisoned by lead, it can only work efficiently on unleaded petrol.

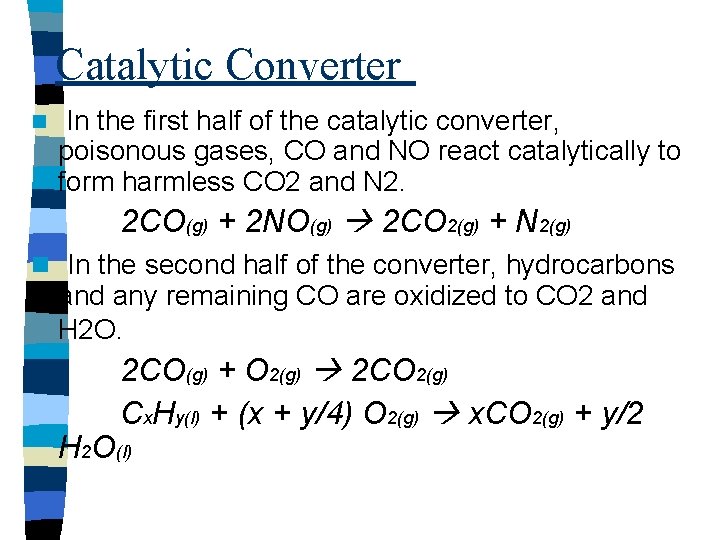
Catalytic Converter n In the first half of the catalytic converter, poisonous gases, CO and NO react catalytically to form harmless CO 2 and N 2. 2 CO(g) + 2 NO(g) 2 CO 2(g) + N 2(g) n In the second half of the converter, hydrocarbons and any remaining CO are oxidized to CO 2 and H 2 O. 2 CO(g) + O 2(g) 2 CO 2(g) Cx. Hy(l) + (x + y/4) O 2(g) x. CO 2(g) + y/2 H 2 O(l)

B. Factories 1. Using cleaner fuels with low sulphur content e. g. diesel fuel 2. Using scrubber -- Acidic sulphur oxide can be removed by reacting with alkalis to form sulphites or sulphates. 3. Using electrostatic precipitators -- Unburned small solid particles can be removed by this method.

Scrubber n A) Dry Scrubber Powdered limestone is added to the hot gases from burning coal. The heat decomposes the limestone to give lime. Ca. CO 3(s) Ca. O(s) + CO 2(g) The lime then react with sulphur dioxide to give calcium sulphite. Ca. O(s) + SO 2(g) Ca. SO 3(g) The calcium sulphite reacts with air to give calcium sulphate. 2 Ca. SO 3(s) + O 2(g) 2 Ca. SO 4(g)

Electrostatic Precipitators The solid particles in the smoke becomes negatively charged when passing through a high voltage electric field. n The negatively charged particles then attracted to the positive electrodes and stick to the sides of the precipitator. n The particles are then shaken to the bottom by the continuous knocking of a steel hammer. n Electrostatic Precipitator are usually used in incinerators and power stations. n