Air pollution MOBILE SOURCES EXHAUST HYDROCARBONS HC CARBON

Air pollution MOBILE SOURCES EXHAUST HYDROCARBONS (HC) CARBON MONOXIDE (CO) NITROGEN OXIDES (NOX) SULFUR COMPOUNDS PM 10
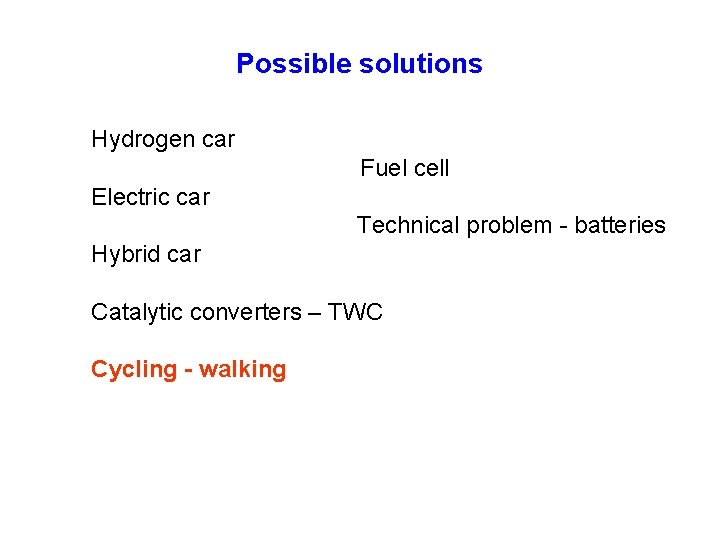
Possible solutions Hydrogen car Fuel cell Electric car Technical problem - batteries Hybrid car Catalytic converters – TWC Cycling - walking

Properties of a suitable Three Way Catalyst Highly active: Conversion > 98%. 50 -100 liters of exhaust to be converted in 1 sec per liter of catalyst. Highly selective: H 2 O, CO 2 and N 2 as products. Thermally stable: working temperature 350 -1100°C. Long life: 200. 000 Km.
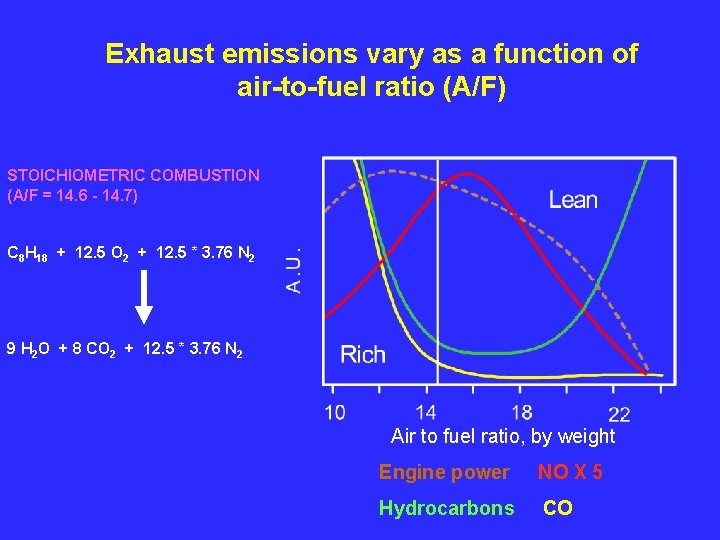
Exhaust emissions vary as a function of air-to-fuel ratio (A/F) STOICHIOMETRIC COMBUSTION (A/F = 14. 6 - 14. 7) C 8 H 18 + 12. 5 O 2 + 12. 5 * 3. 76 N 2 9 H 2 O + 8 CO 2 + 12. 5 * 3. 76 N 2 Air to fuel ratio, by weight Engine power NO X 5 Hydrocarbons CO
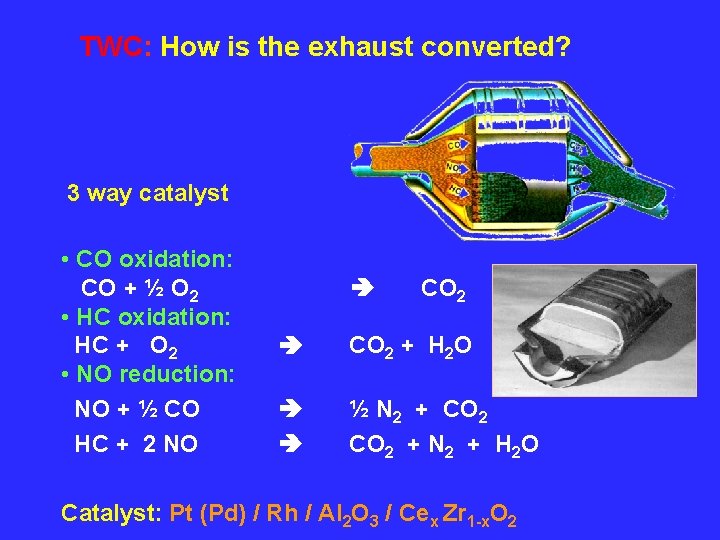
TWC: How is the exhaust converted? 3 way catalyst • CO oxidation: CO + ½ O 2 • HC oxidation: HC + O 2 • NO reduction: NO + ½ CO HC + 2 NO CO 2 + H 2 O ½ N 2 + CO 2 + N 2 + H 2 O Catalyst: Pt (Pd) / Rh / Al 2 O 3 / Cex Zr 1 -x. O 2
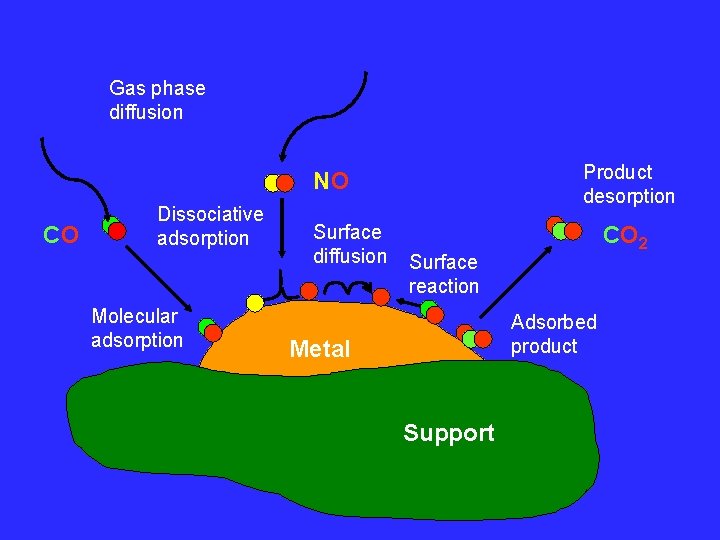
Gas phase diffusion Product desorption NO CO Dissociative adsorption Molecular adsorption Surface diffusion CO 2 Surface reaction Adsorbed product Metal Support
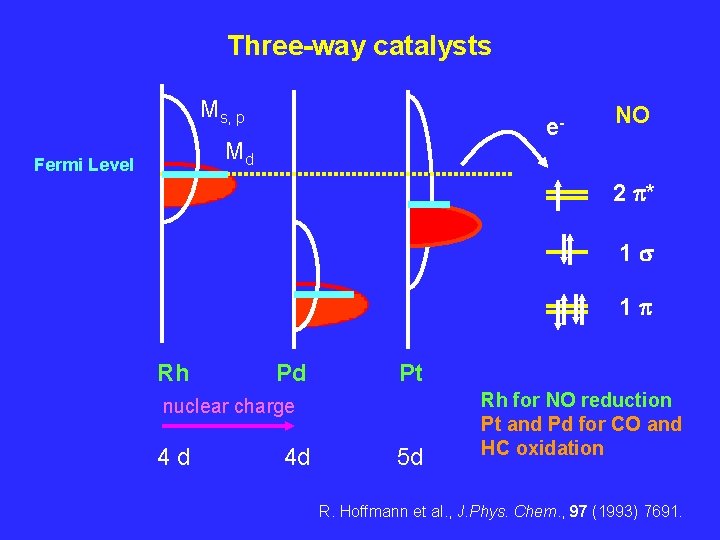
Three-way catalysts Ms, p e- Md Fermi Level NO 2 p* 1 s 1 p Rh Pd Pt nuclear charge 4 d 4 d 5 d Rh for NO reduction Pt and Pd for CO and HC oxidation R. Hoffmann et al. , J. Phys. Chem. , 97 (1993) 7691.

Temperature dependence of conversions Conversion / % 100 CO HC 50 NO 0 473 573 673 Temperature / K 773
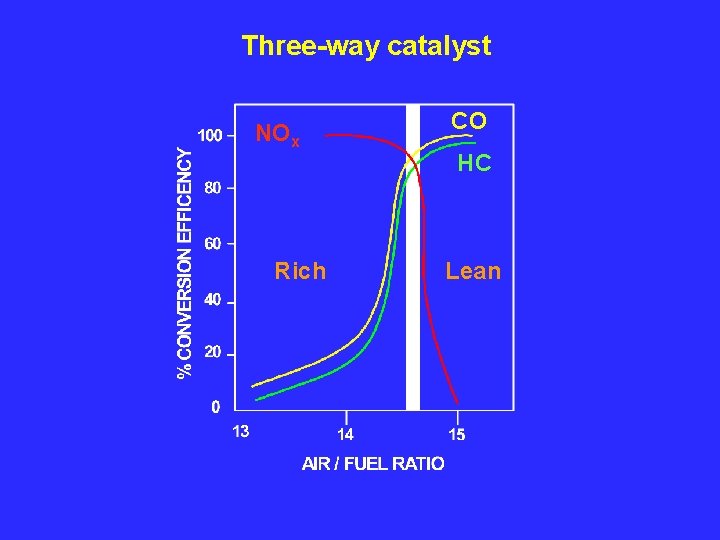
Three-way catalyst NOx Rich CO HC Lean
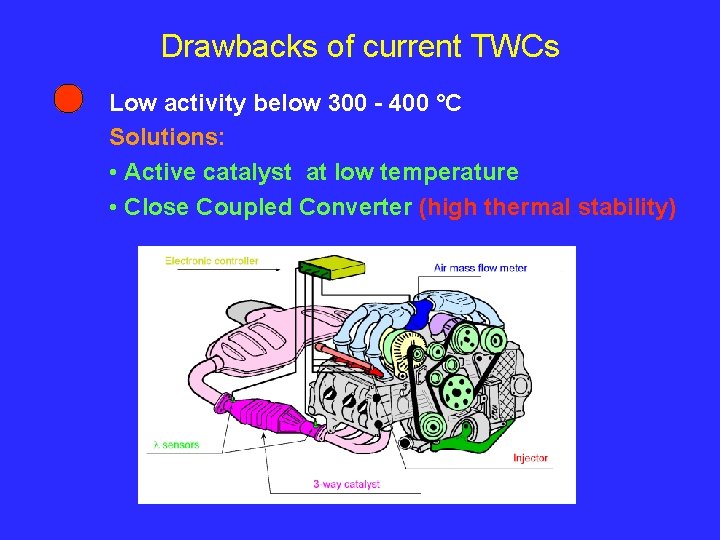
Drawbacks of current TWCs Low activity below 300 - 400 °C Solutions: • Active catalyst at low temperature • Close Coupled Converter (high thermal stability)
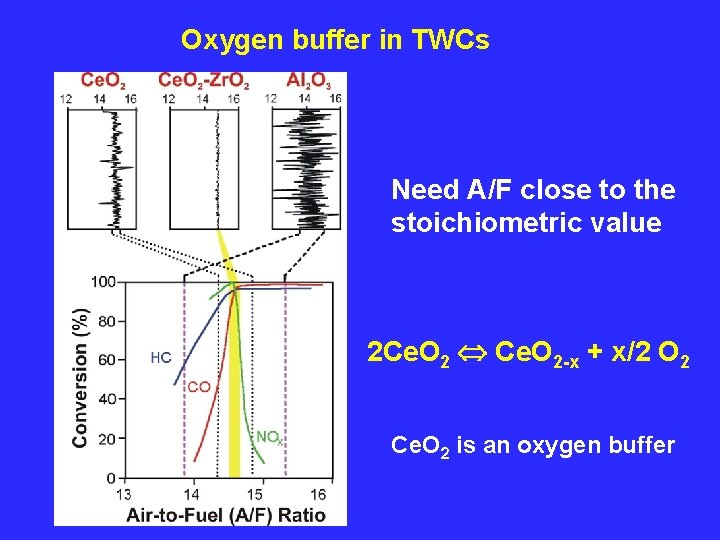
Oxygen buffer in TWCs Need A/F close to the stoichiometric value 2 Ce. O 2 Ce. O 2 -x + x/2 O 2 Ce. O 2 is an oxygen buffer

Why Ce. O 2 -Zr. O 2 solid solutions ? High thermal stability (ceramic materials) incorporation of Ce. O 2 in the solid solution may prevent the undesirable fixation of ceria in the 3+ state such as in Ce. Al. O 3 or Ce 2(CO 3)3.
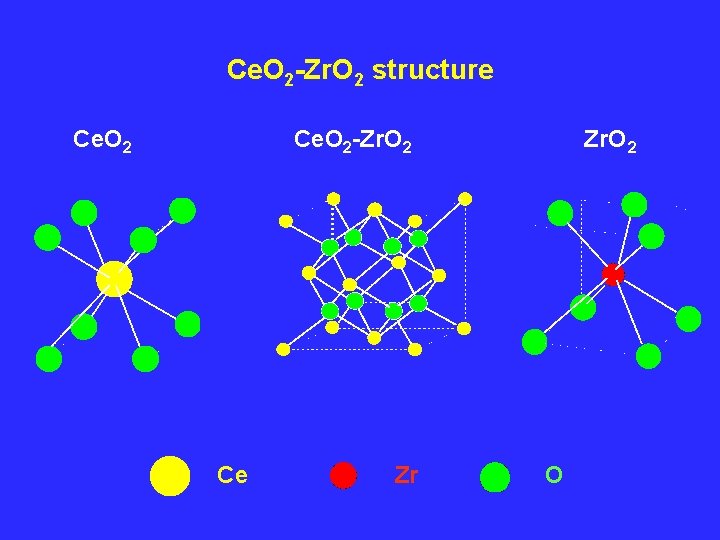
Ce. O 2 -Zr. O 2 structure Ce. O 2 -Zr. O 2 Ce Zr Zr. O 2 O
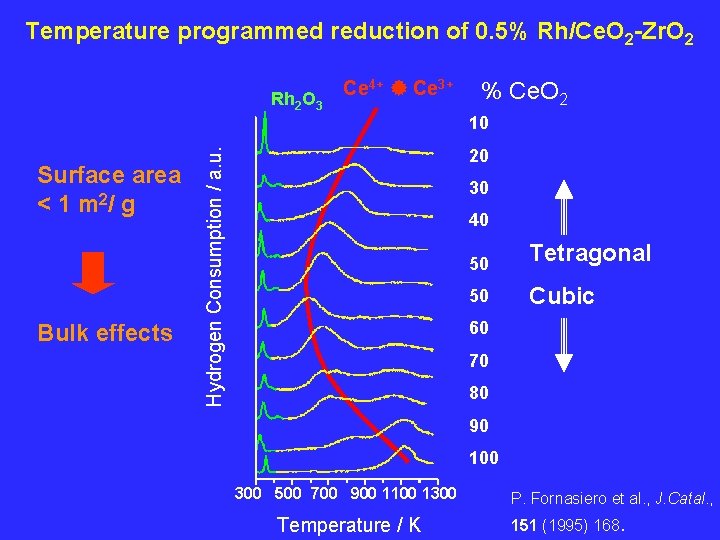
Temperature programmed reduction of 0. 5% Rh/Ce. O 2 -Zr. O 2 Rh 2 O 3 Ce 4+ Ce 3+ % Ce. O 2 10 Bulk effects Hydrogen Consumption / a. u. Surface area < 1 m 2/ g 20 30 40 50 Tetragonal 50 Cubic 60 70 80 90 100 300 500 700 900 1100 1300 Temperature / K P. Fornasiero et al. , J. Catal. , 151 (1995) 168.

Dynamic Oxygen Storage Capacity Both H 2 and CO are present in the exhaust How the dynamic-OSC is affected by the nature of the reducing agent ? What is the importance of surface area in the NM/Ce. O 2 -Zr. O 2 system?
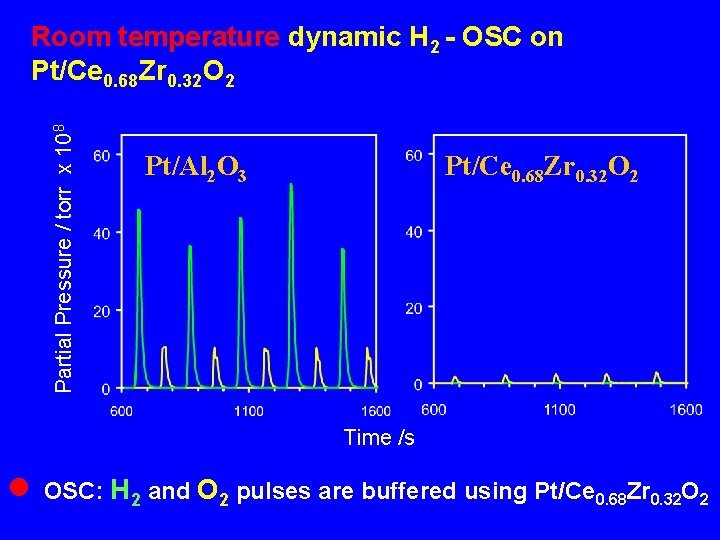
Partial Pressure / torr x 108 Room temperature dynamic H 2 - OSC on Pt/Ce 0. 68 Zr 0. 32 O 2 Pt/Al 2 O 3 Pt/Ce 0. 68 Zr 0. 32 O 2 Time /s n OSC: H 2 and O 2 pulses are buffered using Pt/Ce 0. 68 Zr 0. 32 O 2
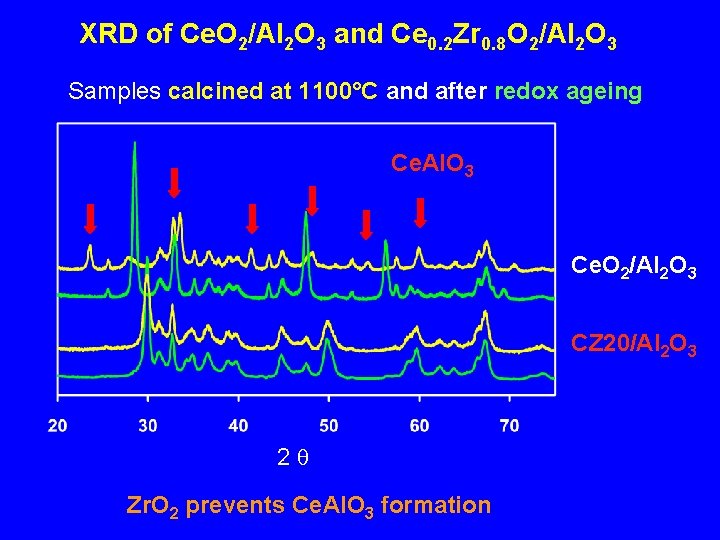
XRD of Ce. O 2/Al 2 O 3 and Ce 0. 2 Zr 0. 8 O 2/Al 2 O 3 Samples calcined at 1100°C and after redox ageing Ce. Al. O 3 Ce. O 2/Al 2 O 3 CZ 20/Al 2 O 3 2 q Zr. O 2 prevents Ce. Al. O 3 formation
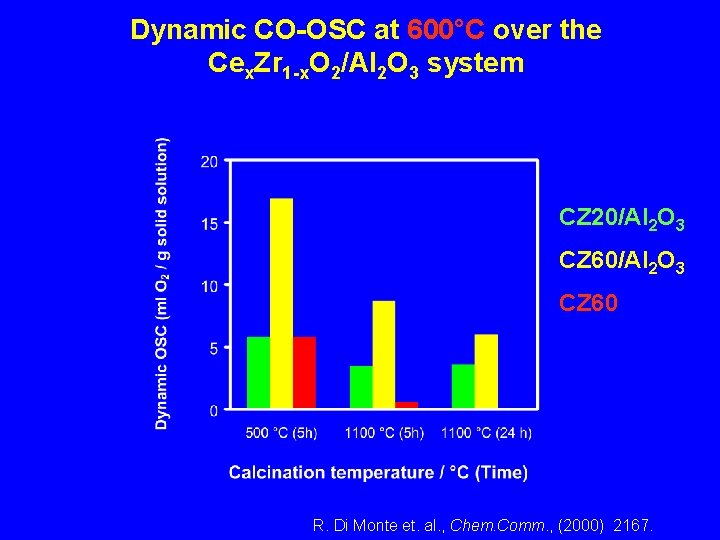
Dynamic CO-OSC at 600°C over the Cex. Zr 1 -x. O 2/Al 2 O 3 system CZ 20/Al 2 O 3 CZ 60 R. Di Monte et. al. , Chem. Comm. , (2000) 2167.
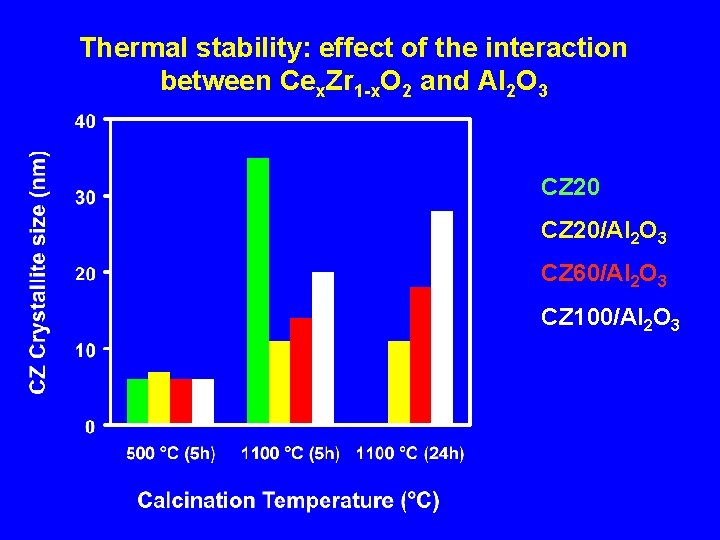
Thermal stability: effect of the interaction between Cex. Zr 1 -x. O 2 and Al 2 O 3 CZ 20/Al 2 O 3 CZ 60/Al 2 O 3 CZ 100/Al 2 O 3
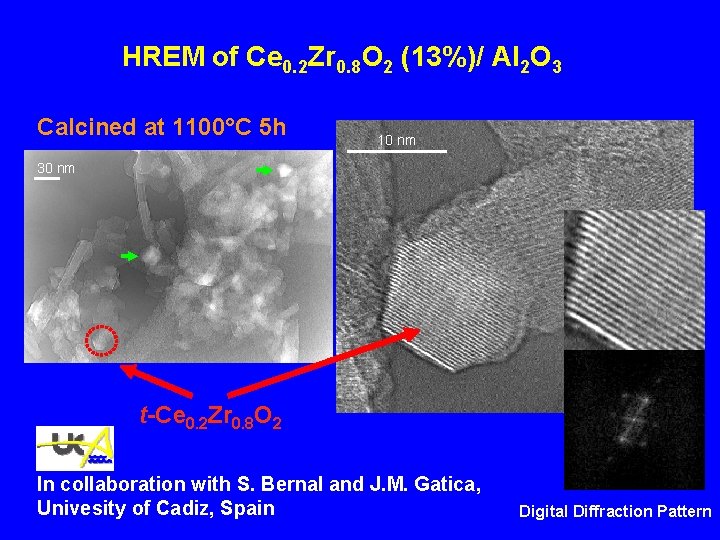
HREM of Ce 0. 2 Zr 0. 8 O 2 (13%)/ Al 2 O 3 Calcined at 1100°C 5 h 10 nm 30 nm t-Ce 0. 2 Zr 0. 8 O 2 In collaboration with S. Bernal and J. M. Gatica, Univesity of Cadiz, Spain Digital Diffraction Pattern
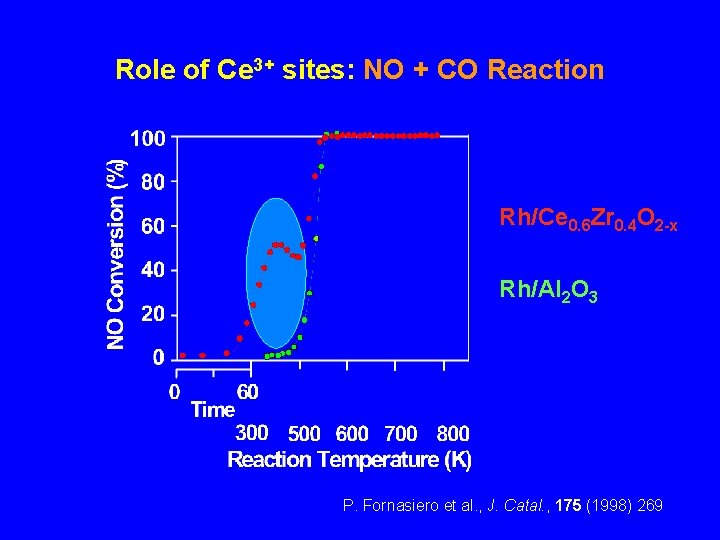
Role of Ce 3+ sites: NO + CO Reaction Rh/Ce 0. 6 Zr 0. 4 O 2 -x Rh/Al 2 O 3 P. Fornasiero et al. , J. Catal. , 175 (1998) 269
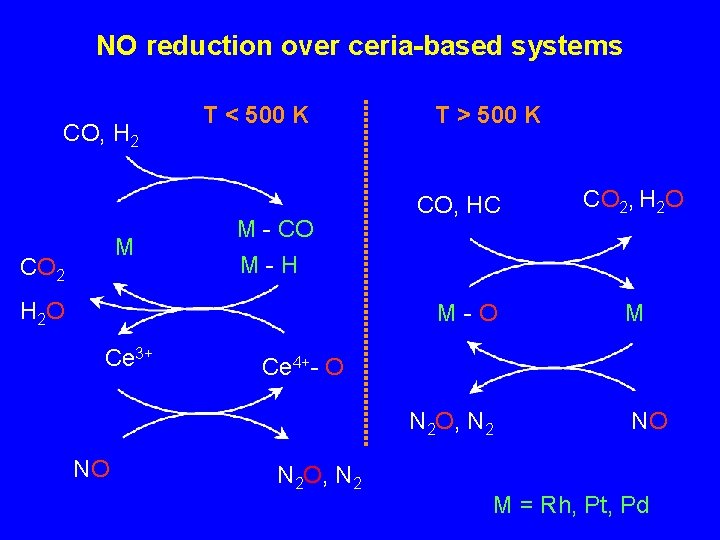
NO reduction over ceria-based systems CO, H 2 M CO 2 T < 500 K M - CO M-H H 2 O T > 500 K CO, HC M-O Ce 3+ M Ce 4+- O N 2 O, N 2 NO CO 2, H 2 O N 2 O, N 2 NO M = Rh, Pt, Pd
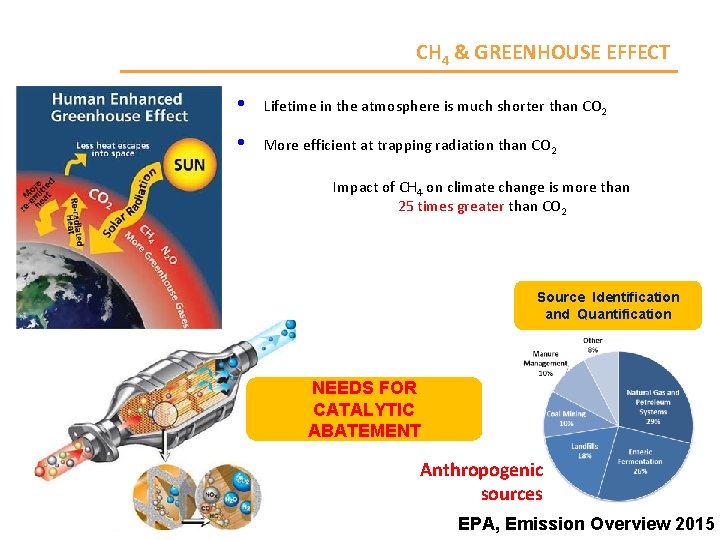
CH 4 & GREENHOUSE EFFECT • Lifetime in the atmosphere is much shorter than CO 2 • More efficient at trapping radiation than CO 2 Impact of CH 4 on climate change is more than 25 times greater than CO 2 Source Identification and Quantification NEEDS FOR CATALYTIC ABATEMENT Anthropogenic sources EPA, Emission Overview 2015
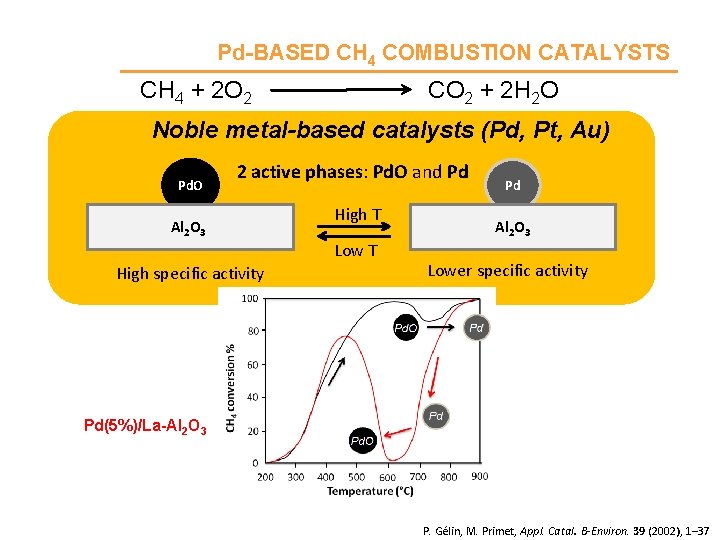
Pd-BASED CH 4 COMBUSTION CATALYSTS CH 4 + 2 O 2 CO 2 + 2 H 2 O Noble metal-based catalysts (Pd, Pt, Au) Pd. O 2 active phases: Pd. O and Pd Al 2 O 3 High T Low T High specific activity Pd Al 2 O 3 Lower specific activity Pd(5%)/La-Al 2 O 3 P. Gélin, M. Primet, Appl. Catal. B-Environ. 39 (2002), 1– 37
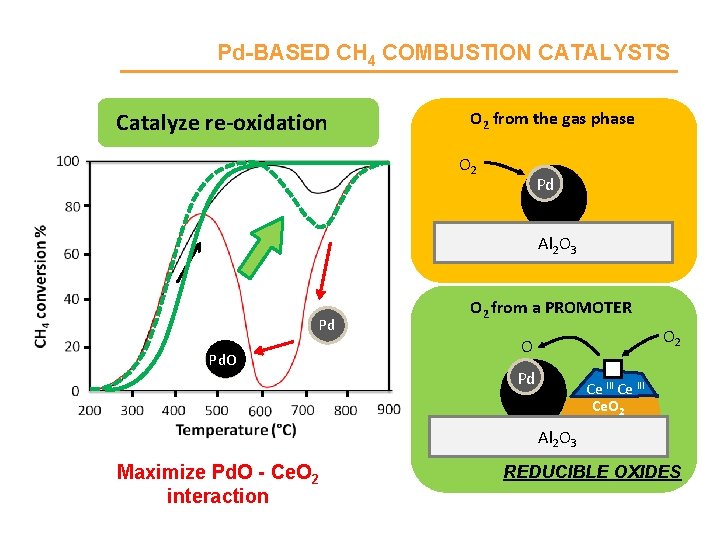
Pd-BASED CH 4 COMBUSTION CATALYSTS Catalyze re-oxidation O 2 from the gas phase O 2 Pd Al 2 O 3 Pd Pd Pd. O O 2 from a PROMOTER O 2 O Pd Ce III Ce. O 2 Al 2 O 3 Maximize Pd. O - Ce. O 2 interaction REDUCIBLE OXIDES
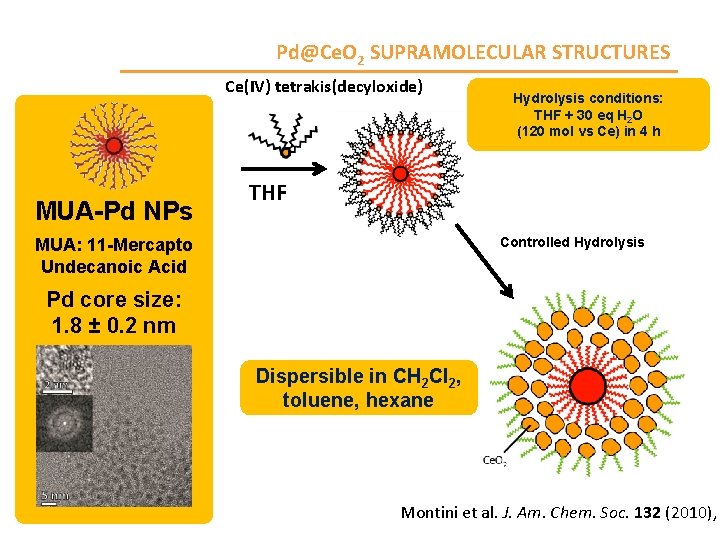
Pd@Ce. O 2 SUPRAMOLECULAR STRUCTURES Ce(IV) tetrakis(decyloxide) MUA-Pd NPs Hydrolysis conditions: THF + 30 eq H 2 O (120 mol vs Ce) in 4 h THF Controlled Hydrolysis MUA: 11 -Mercapto Undecanoic Acid Pd core size: 1. 8 ± 0. 2 nm Dispersible in CH 2 Cl 2, toluene, hexane Montini et al. J. Am. Chem. Soc. 132 (2010),
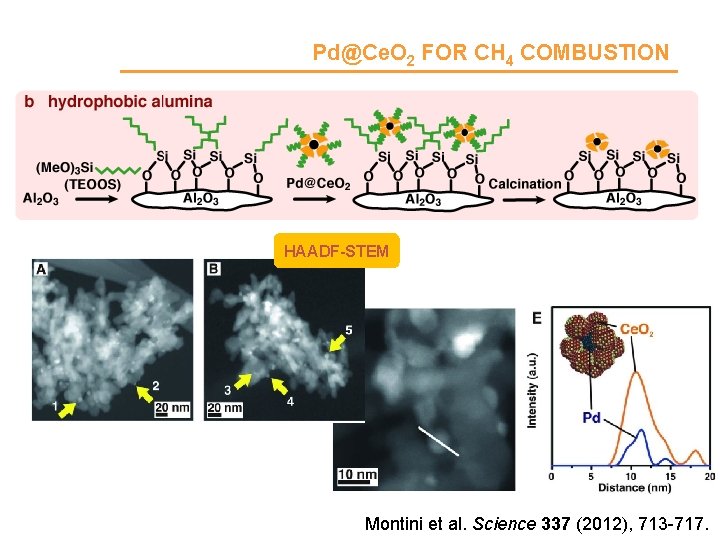
Pd@Ce. O 2 FOR CH 4 COMBUSTION HAADF-STEM Montini et al. Science 337 (2012), 713 -717.
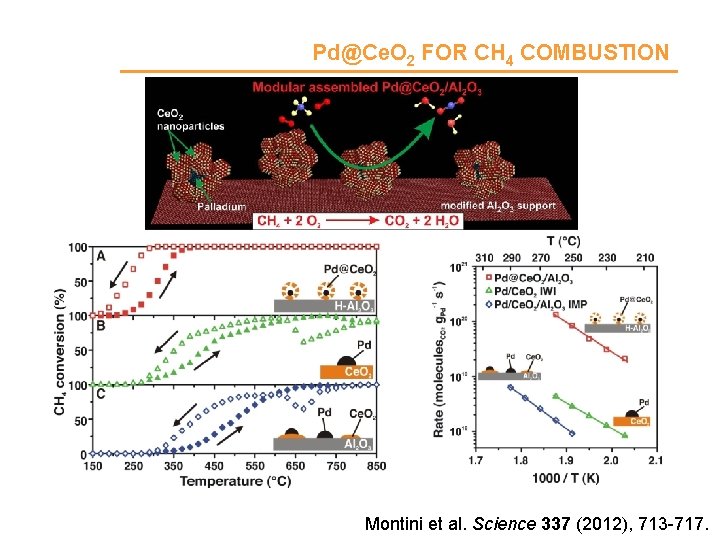
Pd@Ce. O 2 FOR CH 4 COMBUSTION Montini et al. Science 337 (2012), 713 -717.
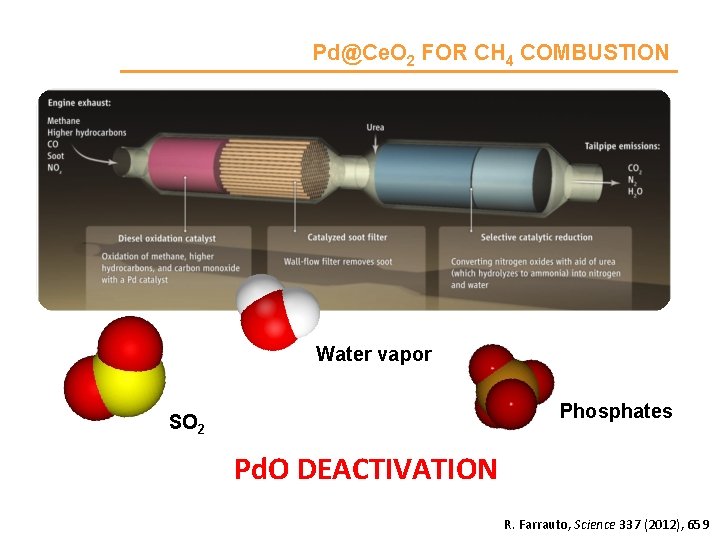
Pd@Ce. O 2 FOR CH 4 COMBUSTION Water vapor Phosphates SO 2 Pd. O DEACTIVATION R. Farrauto, Science 337 (2012), 659
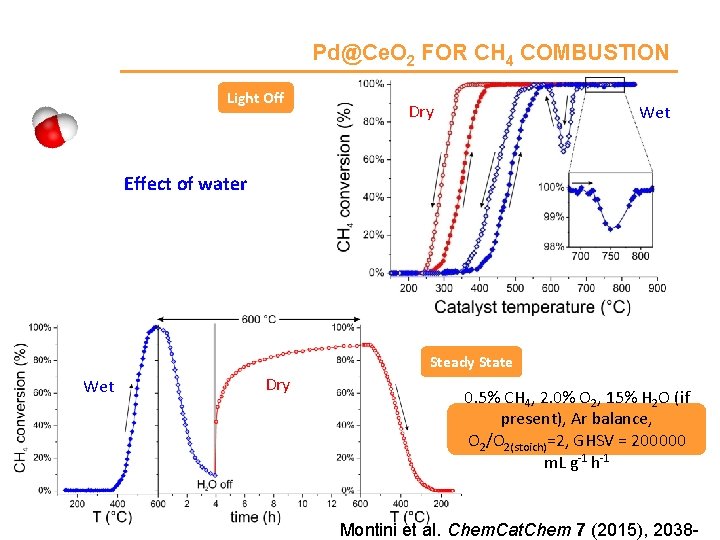
Pd@Ce. O 2 FOR CH 4 COMBUSTION Light Off Dry Wet Effect of water Steady State Wet Dry 0. 5% CH 4, 2. 0% O 2, 15% H 2 O (if present), Ar balance, O 2/O 2(stoich)=2, GHSV = 200000 m. L g-1 h-1 Montini et al. Chem. Cat. Chem 7 (2015), 2038 -
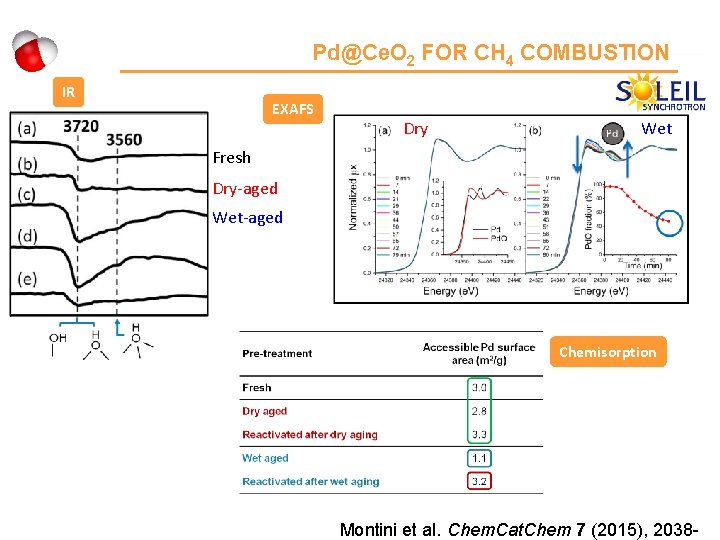
Pd@Ce. O 2 FOR CH 4 COMBUSTION IR EXAFS Dry Wet Fresh Dry-aged Wet-aged Chemisorption Montini et al. Chem. Cat. Chem 7 (2015), 2038 -
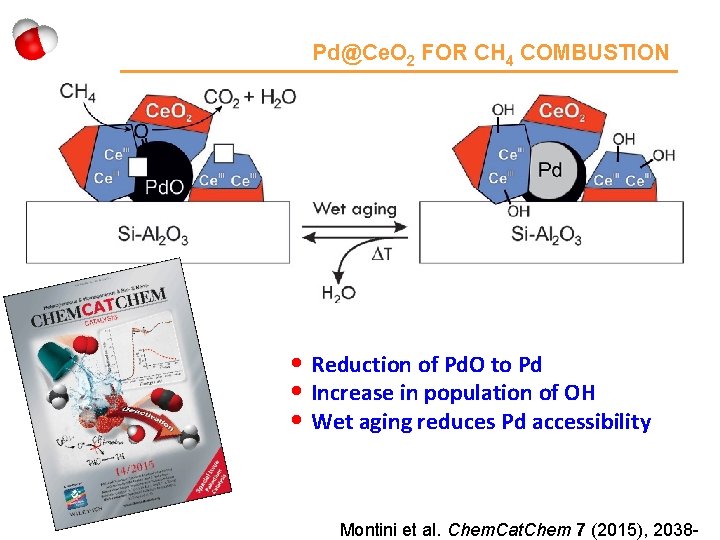
Pd@Ce. O 2 FOR CH 4 COMBUSTION • Reduction of Pd. O to Pd • Increase in population of OH • Wet aging reduces Pd accessibility Montini et al. Chem. Cat. Chem 7 (2015), 2038 -
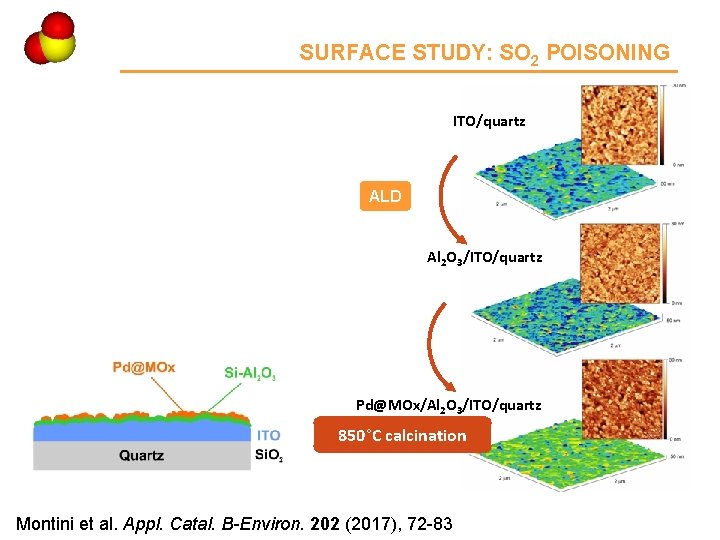
Ce 4 SURFACE STUDY: SO 2 POISONING ITO/quartz ALD Al 2 O 3/ITO/quartz Pd@MOx/Al 2 O 3/ITO/quartz 850°C calcination Montini et al. Appl. Catal. B-Environ. 202 (2017), 72 -83
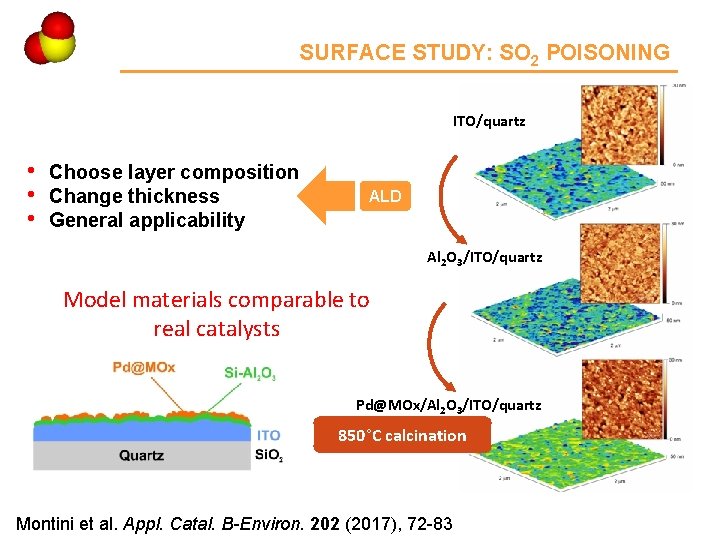
Ce 4 SURFACE STUDY: SO 2 POISONING ITO/quartz • • • Choose layer composition Change thickness General applicability ALD Al 2 O 3/ITO/quartz Model materials comparable to real catalysts Pd@MOx/Al 2 O 3/ITO/quartz 850°C calcination Montini et al. Appl. Catal. B-Environ. 202 (2017), 72 -83
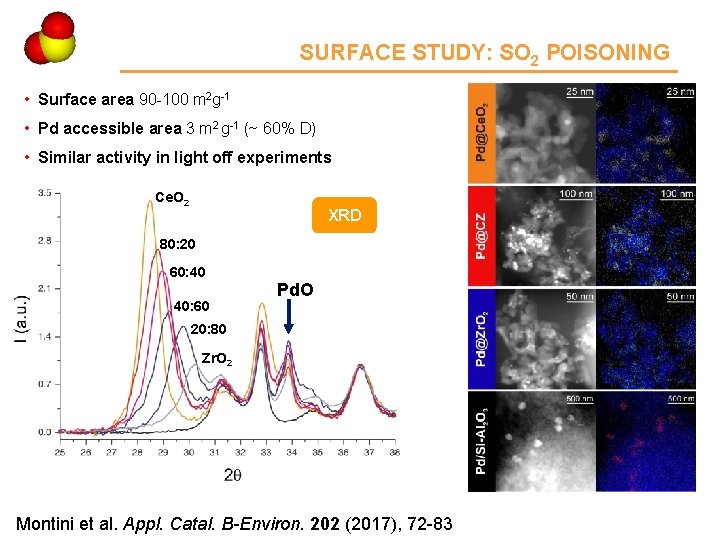
Ce 4 SURFACE STUDY: SO 2 POISONING • Surface area 90 -100 m 2 g-1 • Pd accessible area 3 m 2 g-1 (~ 60% D) • Similar activity in light off experiments Ce. O 2 XRD 80: 20 60: 40 40: 60 Pd. O 20: 80 Zr. O 2 Montini et al. Appl. Catal. B-Environ. 202 (2017), 72 -83
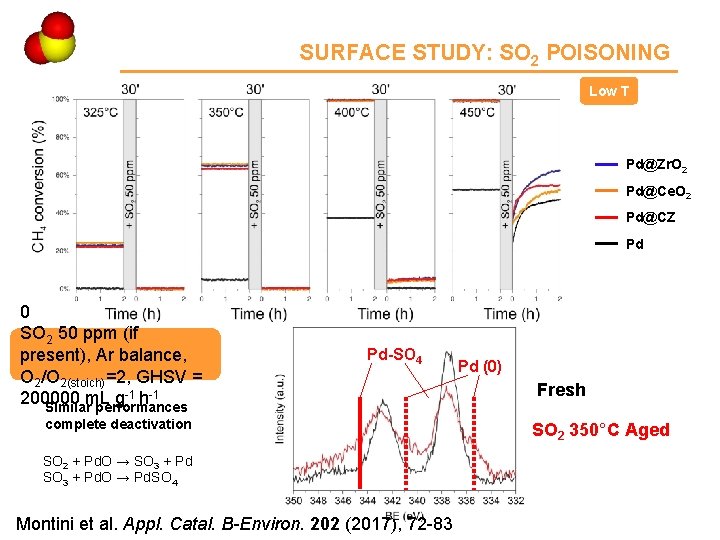
Ce 4 SURFACE STUDY: SO 2 POISONING Low T Pd@Zr. O 2 Pd@Ce. O 2 Pd@CZ Pd 0. 5% CH 4, 2. 0% O 2, SO 2 50 ppm (if present), Ar balance, O 2/O 2(stoich)=2, GHSV = -1 h-1 200000 m. L g Similar performances Pd-SO 4 complete deactivation SO 2 + Pd. O → SO 3 + Pd. O → Pd. SO 4 Montini et al. Appl. Catal. B-Environ. 202 (2017), 72 -83 Pd (0) Fresh SO 2 350°C Aged
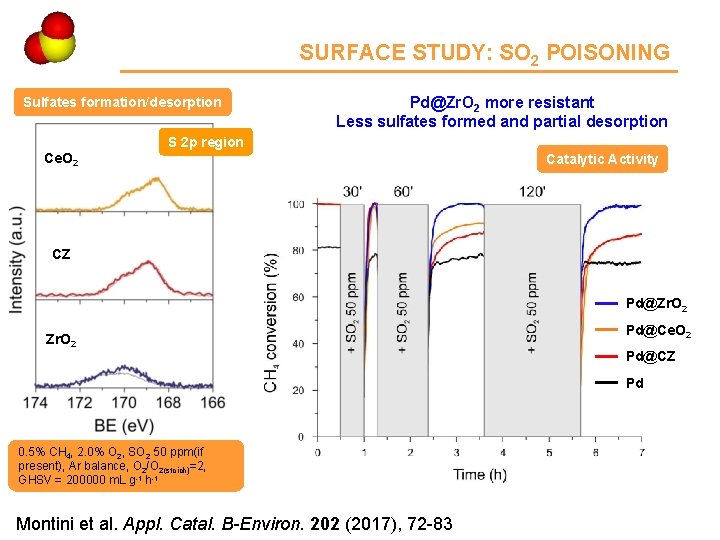
Ce 4 SURFACE STUDY: SO 2 POISONING Sulfates formation/desorption Pd@Zr. O 2 more resistant Less sulfates formed and partial desorption S 2 p region Ce. O 2 Catalytic Activity CZ Pd@Zr. O 2 Pd@Ce. O 2 Pd@CZ Pd 0. 5% CH 4, 2. 0% O 2, SO 2 50 ppm(if present), Ar balance, O 2/O 2(stoich)=2, GHSV = 200000 m. L g-1 h-1 Montini et al. Appl. Catal. B-Environ. 202 (2017), 72 -83
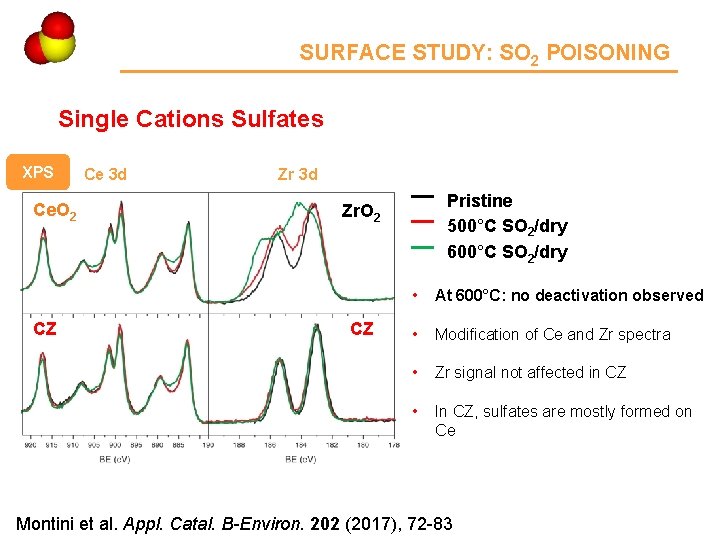
SURFACE STUDY: SO 2 POISONING Single Cations Sulfates XPS Ce. O 2 CZ Ce 3 d Zr 3 d Pristine 500°C SO 2/dry 600°C SO 2/dry Zr. O 2 CZ • At 600°C: no deactivation observed • Modification of Ce and Zr spectra • Zr signal not affected in CZ • In CZ, sulfates are mostly formed on Ce Montini et al. Appl. Catal. B-Environ. 202 (2017), 72 -83
Advantages of Lean technology (Excess of air: A/F > 14. 6) fuel economy (15 -35%) decreased CO 2 emissions low HC and CO emissions Drawback of Lean technology The reduction of NOx is very difficult in the presence of a large excess of O 2 4 NH 3 + 4 NO + O 2 4 N 2 + 6 H 2 O C. Cohn et al. , 1961, US Patent 2, 975, 025.

Selective Catalytic Reduction of NOx with NH 3 4 NH 3 + 4 NO + O 2 4 N 2 + 6 H 2 O 4 NH 3 + 2 NO 2 + O 2 3 N 2 + 6 H 2 O Stationary applications but NOT (yet) for car : Separate and additional supply system Slip of ammonia and on board NH 3 production ? 2 NO + 5 H 2 2 NH 3 + 2 H 2
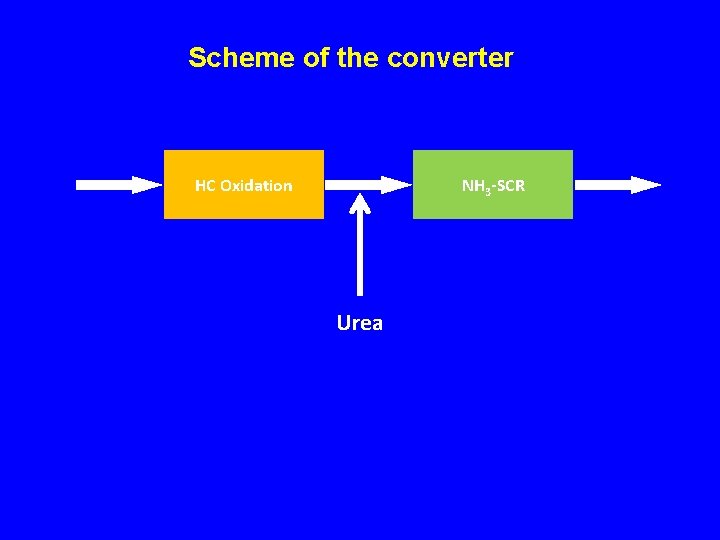
Scheme of the converter HC Oxidation NH 3 -SCR Urea
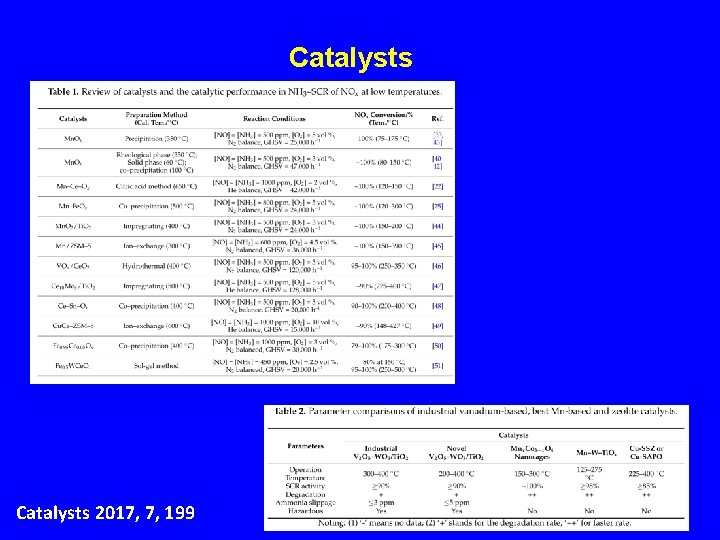
Catalysts 2017, 7, 199
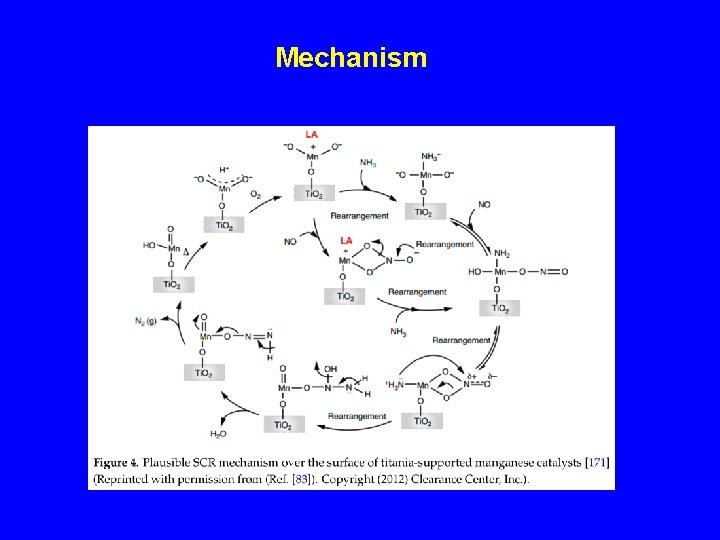
Mechanism
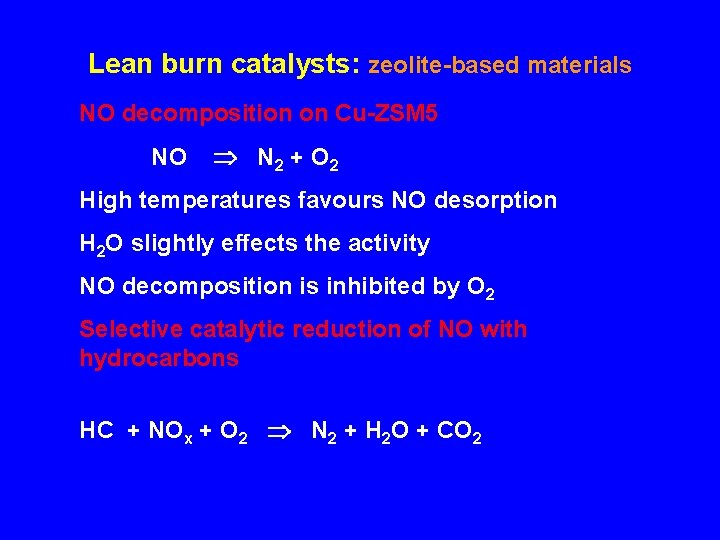
Lean burn catalysts: zeolite-based materials NO decomposition on Cu-ZSM 5 NO N 2 + O 2 High temperatures favours NO desorption H 2 O slightly effects the activity NO decomposition is inhibited by O 2 Selective catalytic reduction of NO with hydrocarbons HC + NOx + O 2 N 2 + H 2 O + CO 2
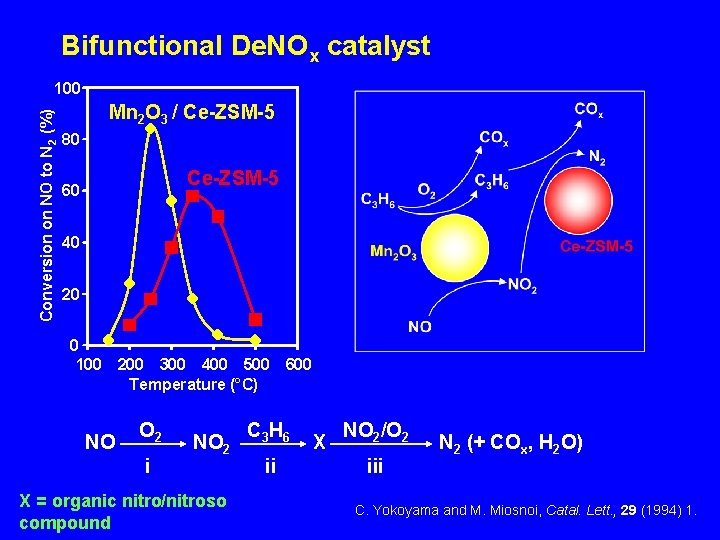
Bifunctional De. NOx catalyst Conversion on NO to N 2 (%) 100 Mn 2 O 3 / Ce-ZSM-5 80 Ce-ZSM-5 60 40 20 0 100 200 300 400 500 600 Temperature (°C) NO O 2 i NO 2 X = organic nitro/nitroso compound C 3 H 6 ii X NO 2/O 2 iii N 2 (+ COx, H 2 O) C. Yokoyama and M. Miosnoi, Catal. Lett. , 29 (1994) 1.
Silver De. NOx catalysts De. NOx: HC combustion: NO + HC O 2 + HC N 2 + CO 2 H 2 O + CO 2 Pt and Pd good oxidation catalyst Growing interest in the use of Ag-based catalysts as possible alternatives Ag/Al 2 O 3 has been widely studied
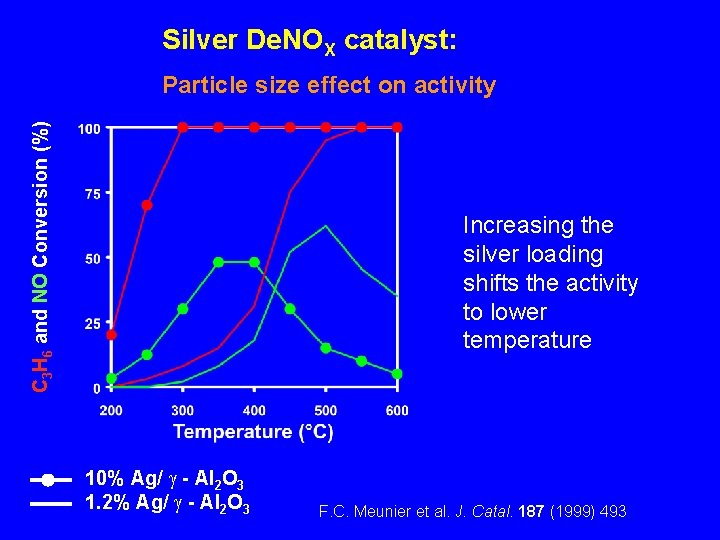
Silver De. NOX catalyst: C 3 H 6 and NO Conversion (%) Particle size effect on activity Increasing the silver loading shifts the activity to lower temperature 10% Ag/ - Al 2 O 3 1. 2% Ag/ - Al 2 O 3 F. C. Meunier et al. J. Catal. 187 (1999) 493.
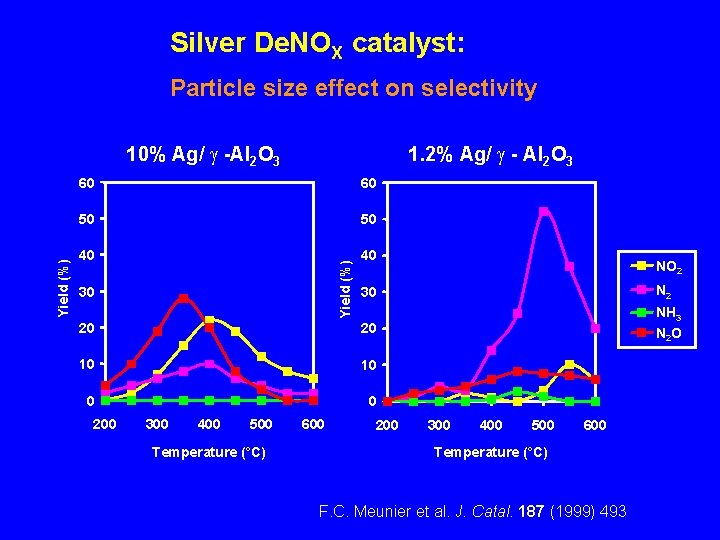
Silver De. NOX catalyst: Particle size effect on selectivity 1. 2% Ag/ - Al 2 O 3 60 60 50 50 40 40 Yield (%) 10% Ag/ -Al 2 O 3 NO 2 30 N 2 20 20 NH 3 N 2 O 10 10 0 0 30 200 300 400 500 Temperature (°C) 600 200 300 400 500 600 Temperature (°C) F. C. Meunier et al. J. Catal. 187 (1999) 493.
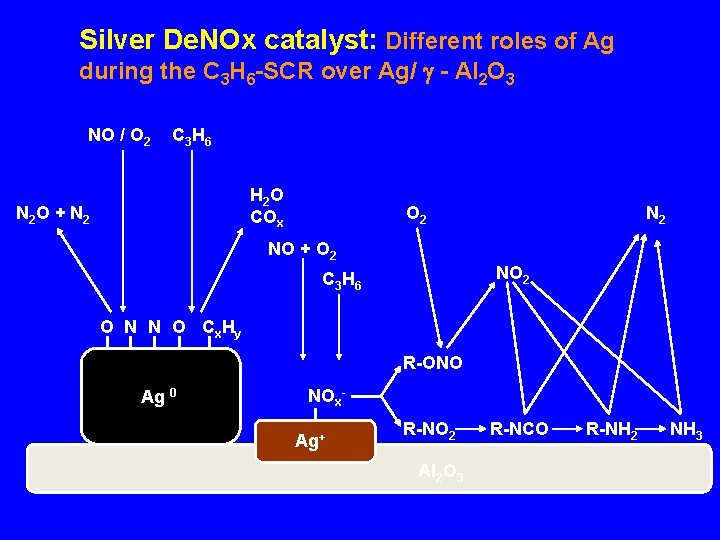
Silver De. NOx catalyst: Different roles of Ag during the C 3 H 6 -SCR over Ag/ - Al 2 O 3 NO / O 2 C 3 H 6 H 2 O COx N 2 O + N 2 O 2 NO + O 2 NO 2 C 3 H 6 O N N O Cx Hy R-ONO Ag 0 NOx. Ag+ R-NO 2 Al 2 O 3 R-NCO R-NH 2 NH 3
Silver De. NOx catalysts There is a support effect on the De-NOx activity of Ag: Both using Zr. O 2 and Ce 0. 16 Zr 0. 84 O 2 as support results in a much lower activity range in comparison with Al 2 O 3. Ag/Zr. O 2 deactivates completely in the presence of SO 2 but it can be easily regenerated. Ag/Al 2 O 3 deactivates partially in the presence of SO 2 and it is not easily regenerated. Ag/Ce 0. 16 Zr 0. 84 O 2 deactivates partially in the presence of SO 2 and it can be easily regenerated.

“ The Toyota” concept None of the studied system show acceptable activity, hydrothermal stability or resistance to sulphur. So, perhaps it is not possible to achieve the goal of efficiently reducing NOx in the car exhaust under oxidising condition.
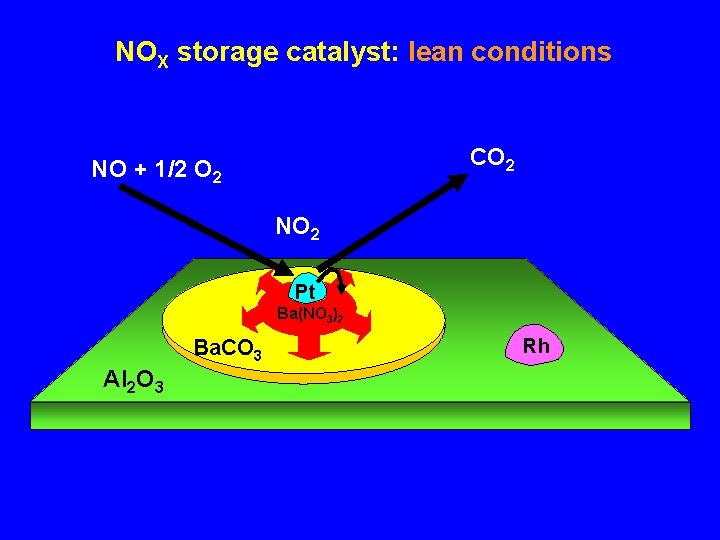
NOX storage catalyst: lean conditions CO 2 NO + 1/2 O 2 NO 2 Pt Ba(NO 3)2 Ba. CO 3 Al 2 O 3 Rh
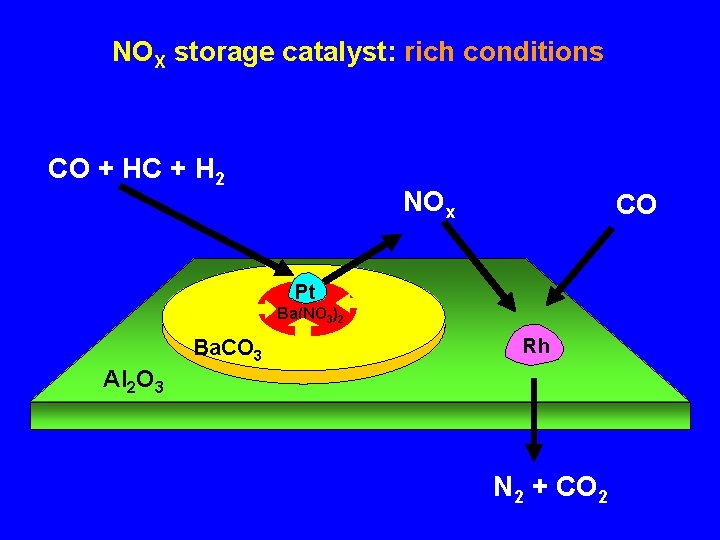
NOX storage catalyst: rich conditions CO + HC + H 2 NOx CO Pt Ba(NO 3)2 Ba. CO 3 Rh Al 2 O 3 N 2 + CO 2
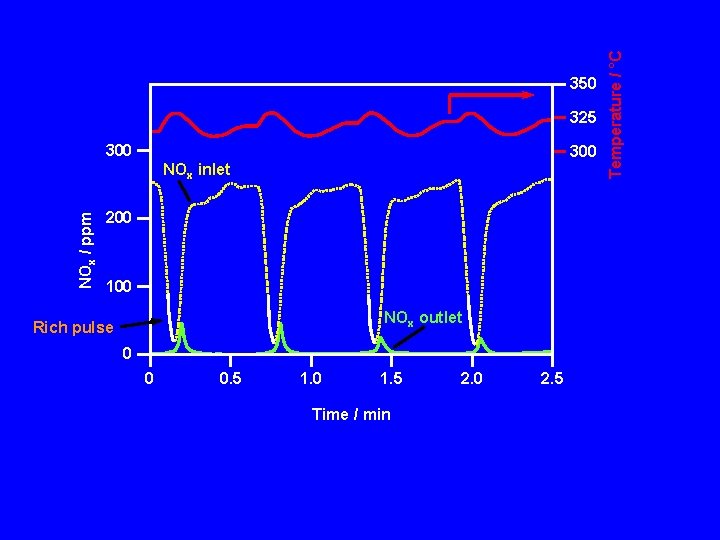
325 300 NOx / ppm NOx inlet 200 100 NOx outlet Rich pulse 0 0 0. 5 1. 0 1. 5 Time / min 2. 0 2. 5 Temperature / °C 350
- Slides: 55