Air pollution control methods Introduction Air pollution control











- Slides: 19

Air pollution control methods

Introduction • Air pollution control can be generally described as a “separation” technology. • The pollutants, whether they are gaseous, aerosol, or solid particulate, are separated from a carrier gas, which is usually air. • We separate these substances because, these pollutants may adversely affect our health and that of the environment.

Treatment of air 1. separation of particles 2. separation of gases

1. separation of particles a) Dry collection of particles b) Wet collection of particles

a) Dry collection of particulate • Such as fabric filter collectors (baghouses) and electrostatic precipitators • Baghouses are often precoated with a fine material to reduce the permeability of the collecting filter cake and improve fine particulate capture. • This cake adds to the pressure drop but an increase in energy input. • Precipitators are often increased in field size to remove finer particulate thereby requiring greater power input. • Dry devices use less total power input than equivalent wet devices when removing particulate.

• For very large particles (those greater than approximately 50 µm aerodynamic diameter or about the diameter of a human hair), traps, and knock out chambers are used. • These basically slow the gas stream down sufficiently so that the particles drop out. • These are often seen on the end of lime kilns and mineral calciners as primary separators.

b) Wet collection of particulate • Wet scrubbers remove particulate by shooting the particulate at target droplets of liquid. • Once the particulate is into the droplet, water droplets generally tend to agglomerate and increase in size upon contact. • If we spin, impact, or compress the droplets together, they combine to form even easier to remove droplets. • Wet scrubbers exhibit an increase in total energy input as the target particle size decreases as a result of the capture technique used. • Particles greater than approximately 2 to 5µm tend to behave more like gases and follow a given path. • Particles less than approximately 2µ m diameter tend to be influenced by gas molecules, temperature and density gradients, and other forces and do not follow predictable trajectories.

Capture mechanisms of Wet collection • Impaction • Interception ( )ﺑﺮﺧﻮﺭﺩ کﺮﺩﻥ • Diffusion

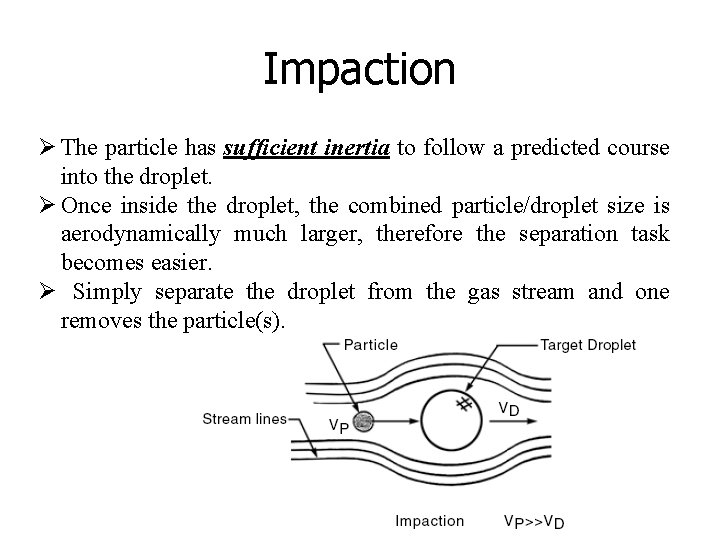
Impaction Ø The particle has sufficient inertia to follow a predicted course into the droplet. Ø Once inside the droplet, the combined particle/droplet size is aerodynamically much larger, therefore the separation task becomes easier. Ø Simply separate the droplet from the gas stream and one removes the particle(s).

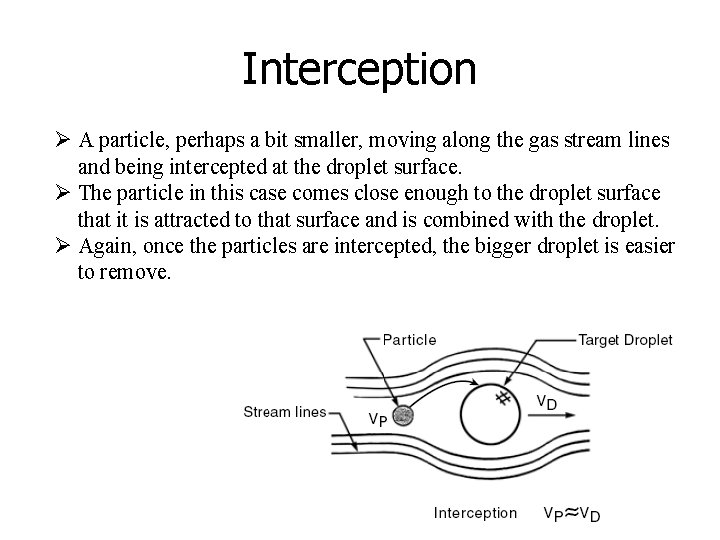
Interception Ø A particle, perhaps a bit smaller, moving along the gas stream lines and being intercepted at the droplet surface. Ø The particle in this case comes close enough to the droplet surface that it is attracted to that surface and is combined with the droplet. Ø Again, once the particles are intercepted, the bigger droplet is easier to remove.

Diffusion Ø a tinier particle that is so small it bounces around in the moving air stream affeted by water and gas molecules. Ø The particle diffuses over to the droplet and, by chance, is absorbed into the droplet. Ø To increase the chances of capture by diffusion, increase the number of droplets per unit volume. Ø the smaller the target droplet and the closer the droplet is to an adjacent droplet, the greater the percentage particulate capture. Ø To make greater quantities of smaller droplets requires increased energy input to shear Ø The higher the velocity out of the nozzle, the finer the spray.

2. separation of contaminant gases • The processes involved in the separation of contaminant gases from a carrier gas include: a. Condensation b. Absorption c. Adsorption d. Gas phase destruction (thermal or chemical)

a. Condensation Ø cooling the gas stream sufficiently to condense the contaminant gas Ø the resulting outlet gas stream may still contain the amount of contaminant gas that will be at equilibrium with the carrier gas Ø Condensation is therefore useful but not always totally effective unless one cools the carrier gas to very low temperatures.

b. Absorption • the most common mechanism used in the control of contaminant gases. • Absorption is maximized by: 1. Creating and maintaining the highest liquid surface area to unit gas volume as possible 2. Creating and maintaining a favorable concentration gradient in the scrubbing liquid vs. the contaminant gas • Contaminant gases can only enter a liquid stream at a given number of molecules per unit area. This varies by the type of contaminant, the type of liquid, the temperature, solubility, and other parameters. • The greater the surface area of liquid, the greater the amount of gas that can be absorbed and the greater the rate at which it can be absorbed.

c. Adsorption • Adsorption is a separation process where the contaminant gas becomes physically attached to a medium, usually activated carbon, zeolites, or clays. • The contaminant gas is physically attached to the adsorbent’s surface or in pores. • Because the pollutant is physically attached, conditions can often be applied that desorb the pollutant from the adsorbent. • Because water vapor can be adsorbed by many of the activated carbon products, water vapor is typically removed prior to an adsorber using carbon.

d. Gas phase destruction • In these devices, the chemical bonds of the pollutant are broken through the application of heat, electrical, or light energy. • Gas phase destruction generally occurs in devices called thermal oxidizers. • These devices typically contain: 1. a burner that, at least, preheats and initiates thermal oxidation process 2. a chamber or housing that contains the products of combustion long enough to allow the desired destruction of the pollutant. • For burning solid or mixed wastes, the solid wastes may be first volatilized or converted to carbon, then oxidized in an afterburner. The afterburner becomes the first stage, in effect, of an air pollution control system. This arrangement is common for medical and hazardous waste incinerator systems.

• At present, other technologies such as the application of intense ultraviolet (UV) light are beginning to be explored. • In systems that use UV light, an oxidant (such as hydrogen peroxide) is typically injected into a mixed gas stream followed by the application of intense UV light. • The hydroxyl radicals formed by breaking the oxygen/hydrogen bond in the peroxide rather than using free oxygen present in the gas stream attack the pollutant.

Commonly Used Methods For Air Pollution Control PARTICULATE · Cyclones · Electrostatic Precipitators · Fabric Filter · Wet Scrubbers GASES · Adsorption Towers · Thermal Incernation · Catalytic Combustion

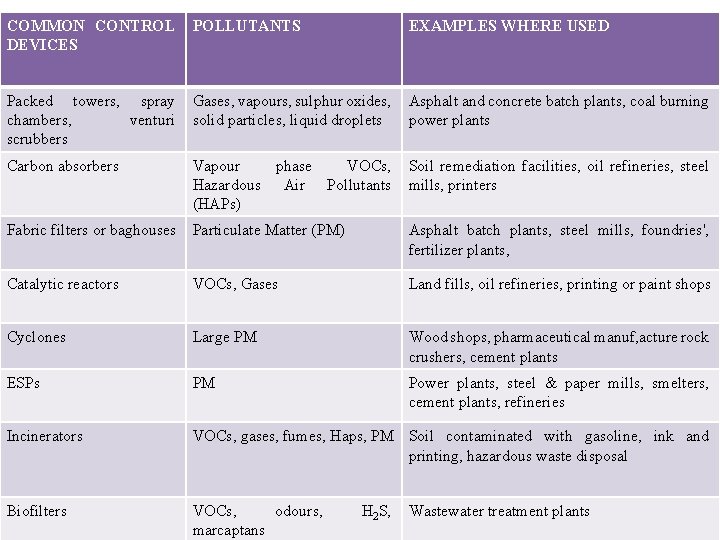
COMMON CONTROL DEVICES POLLUTANTS EXAMPLES WHERE USED Packed towers, spray chambers, venturi scrubbers Gases, vapours, sulphur oxides, solid particles, liquid droplets Asphalt and concrete batch plants, coal burning power plants Carbon absorbers Vapour phase VOCs, Hazardous Air Pollutants (HAPs) Soil remediation facilities, oil refineries, steel mills, printers Fabric filters or baghouses Particulate Matter (PM) Asphalt batch plants, steel mills, foundries', fertilizer plants, Catalytic reactors VOCs, Gases Land fills, oil refineries, printing or paint shops Cyclones Large PM Wood shops, pharmaceutical manuf, acture rock crushers, cement plants ESPs PM Power plants, steel & paper mills, smelters, cement plants, refineries Incinerators VOCs, gases, fumes, Haps, PM Soil contaminated with gasoline, ink and printing, hazardous waste disposal Biofilters VOCs, odours, marcaptans H 2 S, Wastewater treatment plants