Aim What is hybridization of the orbitals Announcements

Aim: What is hybridization of the orbitals? Announcements: Reading Guide # 5 due Monday Lab # 5 Monday
MOLECULAR SHAPES: VALENCE BOND THEORY (VBT) • Valence Bond Theory: a quantum mechanical description of bonding that pictures covalent bond formation as the overlap of two singly occupied atomic orbitals. • Hybridization varies from sp, sp 2, up to sp 3 d 2 depending upon the number of orbitals involved in the bonding. • Each of these has a characteristic shape • Hybridization determined by using VSEPR to establish the geometry, i. e. , the number of electron clouds around the central atom. The number of electron clouds = the number of hybrid orbitals.

Hybridization • sp hybridization – results from the overlap of an s orbital with one p orbital. The bond formed has 180 degree angle and the molecule is linear. • sp² hybridization – results from the overlap of an s orbital with two p orbitals. Three sp² orbitals have trigonal planar orientation and a bond of 120 degrees. • sp³ hybridization – results from the overlap of an s orbit with 3 p orbits. The molecule will have a tetrahedral geometry. • sp³d hybridization – results from the blending of an s orbital, 3 p orbitals and one d orbital. This molecule will have a trigonal bipyramidal geometry. • sp³d² hybridization – occurs when one s, three p and two d orbitals are mixed giving an octahedral geometry.
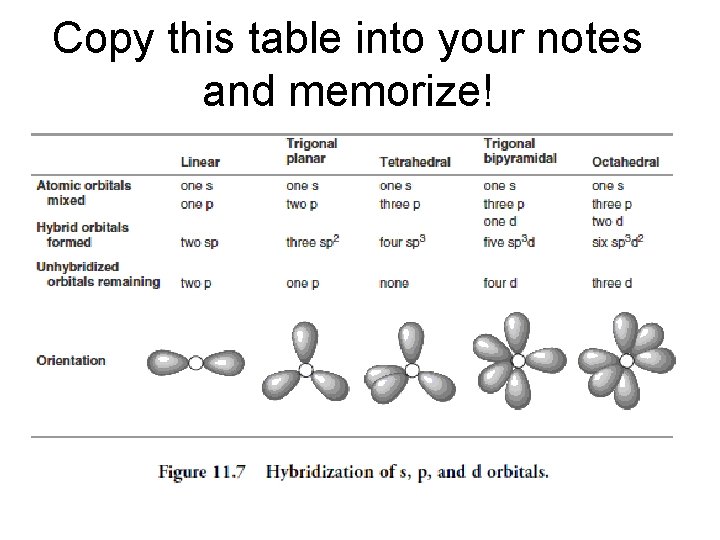
Copy this table into your notes and memorize!
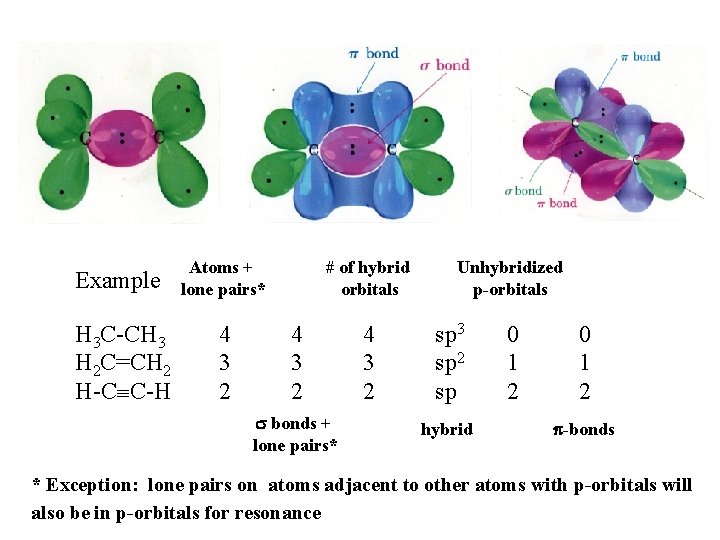
Example H 3 C-CH 3 H 2 C=CH 2 H-CºC-H Atoms + lone pairs* 4 3 2 # of hybrid orbitals 4 3 2 s bonds + lone pairs* 4 3 2 Unhybridized p-orbitals sp 3 sp 2 sp hybrid 0 1 2 p-bonds * Exception: lone pairs on atoms adjacent to other atoms with p-orbitals will also be in p-orbitals for resonance
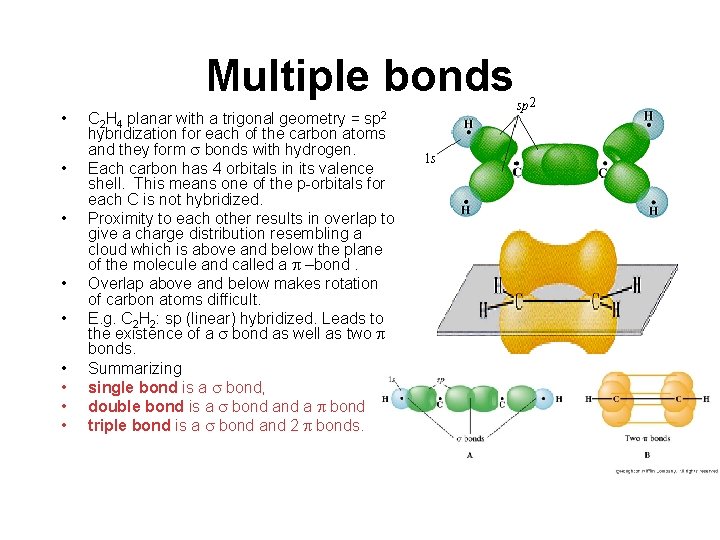
Multiple bonds • • • C 2 H 4 planar with a trigonal geometry = sp 2 hybridization for each of the carbon atoms and they form bonds with hydrogen. Each carbon has 4 orbitals in its valence shell. This means one of the p-orbitals for each C is not hybridized. Proximity to each other results in overlap to give a charge distribution resembling a cloud which is above and below the plane of the molecule and called a –bond. Overlap above and below makes rotation of carbon atoms difficult. E. g. C 2 H 2: sp (linear) hybridized. Leads to the existence of a bond as well as two bonds. Summarizing single bond is a bond, double bond is a bond, triple bond is a bond and 2 bonds.
MO Theory of Bonding • Molecular Orbital Theory extends quantum theory and states that electrons spread throughout the molecule in molecular orbitals = region in a molecule in which an electron is likely to be which is similar to the concept discussed in quantum theory. Molecular orbitals are considered to be the result of the combination of atomic orbitals. • HOMO – Highest occupied molecular orbitals • LUMO – Lowest unoccupied molecular orbital
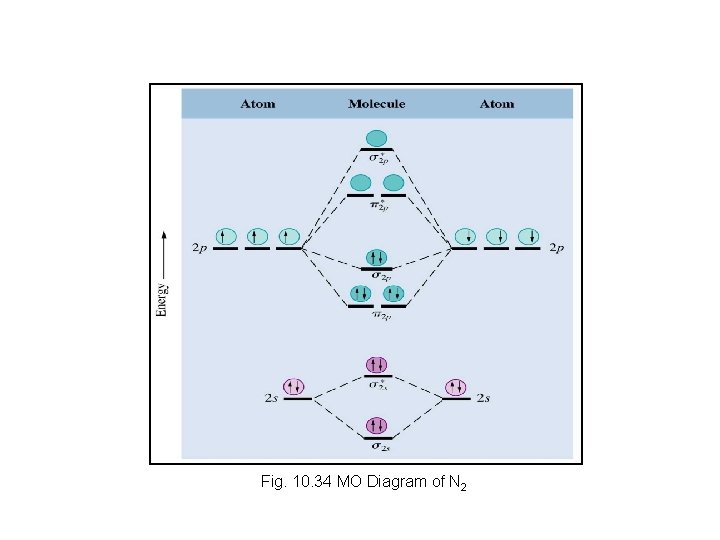
Fig. 10. 34 MO Diagram of N 2
• BONDING AND ANTIBONDING ORBITALS • Bonding molecular orbital (σ or π) - is of lower energy than the two 1 s atomic orbitals making this orbital more stable than two separated atomic orbitals. • The upper molecular orbital has a node in the electronic wave function and the electron density is low between the two positively charged nuclei. The energy of the upper orbital is greater than that of the 1 s atomic orbital, and such an orbital is called an antibonding molecular orbital (σ* or π*).
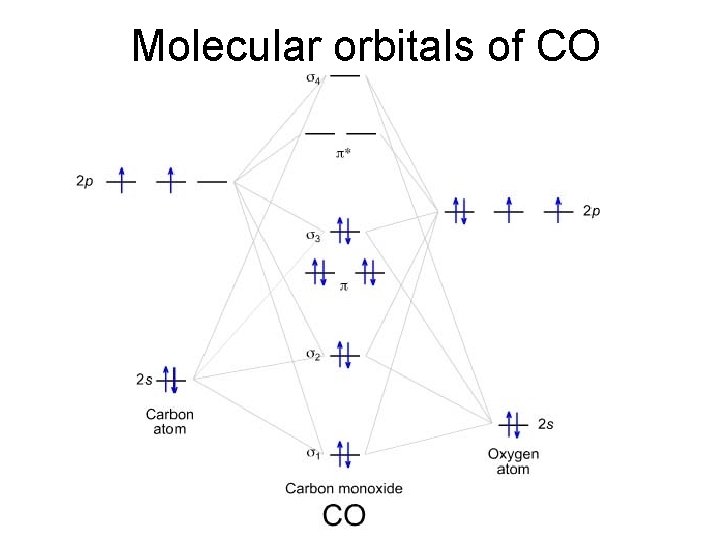
Molecular orbitals of CO

Molecular Orbitals of F 2

Molecular Bonding of O 2

Let’s have you try to do this one • Nitrogen monoxide
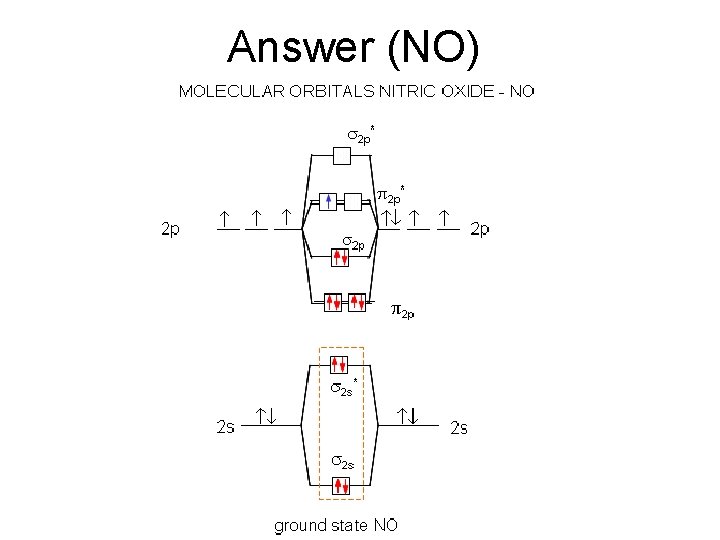
Answer (NO)
Practice molecular diagrams for the following molecules • • • C and Cl C and H C and N Br and C Li and Cl
- Slides: 15