Aim What is equilibrium and how can a

Aim: What is equilibrium and how can a stress effect equilibrium?
Irreversible vs Reversible Reactions • In a irreversible reaction ( ), the reactants react to form the products, which cannot change back into reactants • A reversible reaction ( ) is one in which the conversion of reactants to products and the conversion of products to reactants occur simultaneously (at the same time).
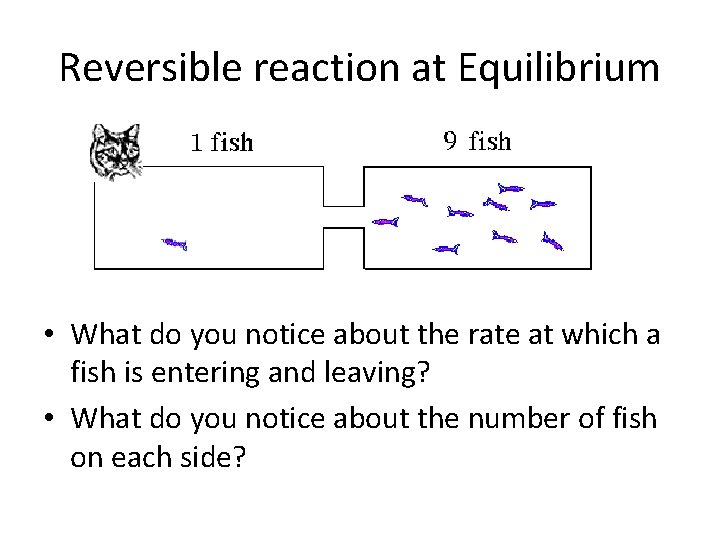
Reversible reaction at Equilibrium • What do you notice about the rate at which a fish is entering and leaving? • What do you notice about the number of fish on each side?

Equilibrium • At dynamic equilibrium, reactants are converted to products and products are converted to reactants at an equal and constant rate • Equilibrium occurs when – the forward and reverse reactions occur at the same rate; – the concentration of the reactants and products remain constant. • Can only occur in a closed system; reactants nor products can leave the system.
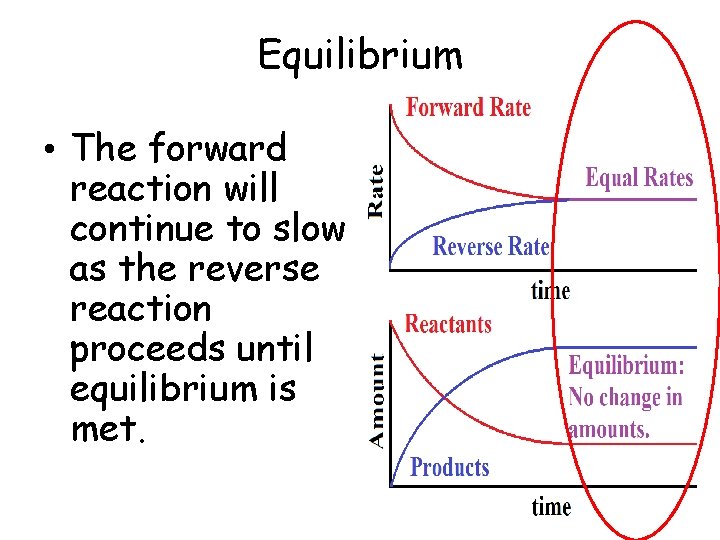
Equilibrium • The forward reaction will continue to slow as the reverse reaction proceeds until equilibrium is met.
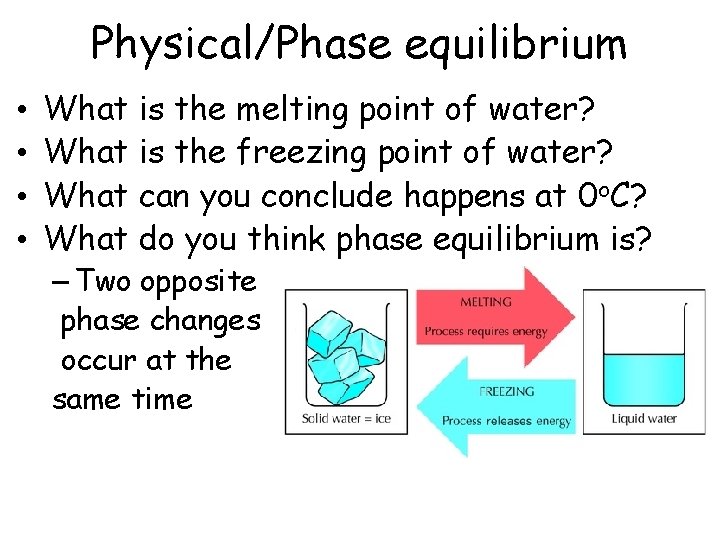
Physical/Phase equilibrium • • What is the melting point of water? What is the freezing point of water? What can you conclude happens at 0 o. C? What do you think phase equilibrium is? – Two opposite phase changes occur at the same time
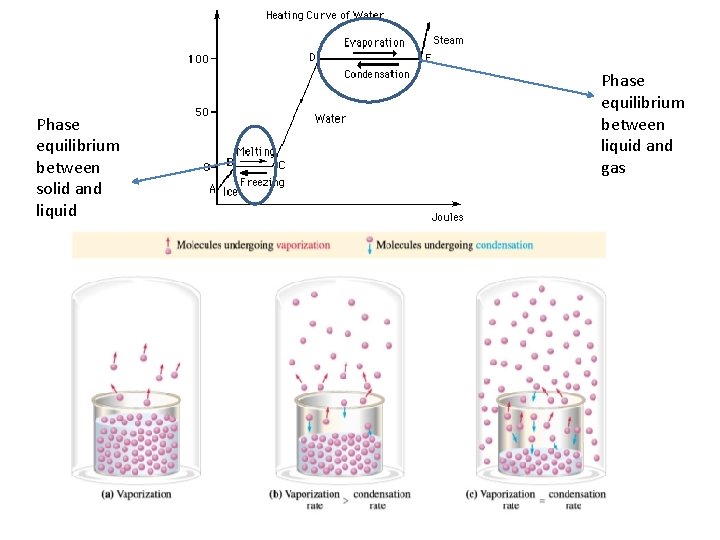
Phase equilibrium between solid and liquid Phase equilibrium between liquid and gas
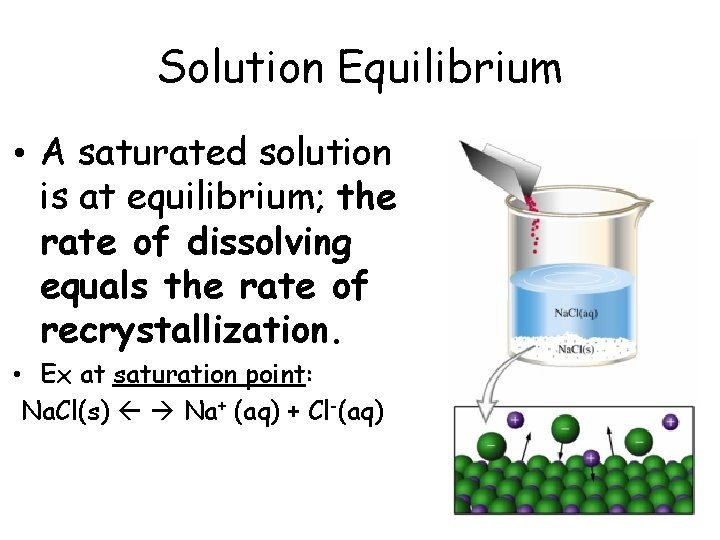
Solution Equilibrium • A saturated solution is at equilibrium; the rate of dissolving equals the rate of recrystallization. • Ex at saturation point: Na. Cl(s) Na+ (aq) + Cl-(aq)
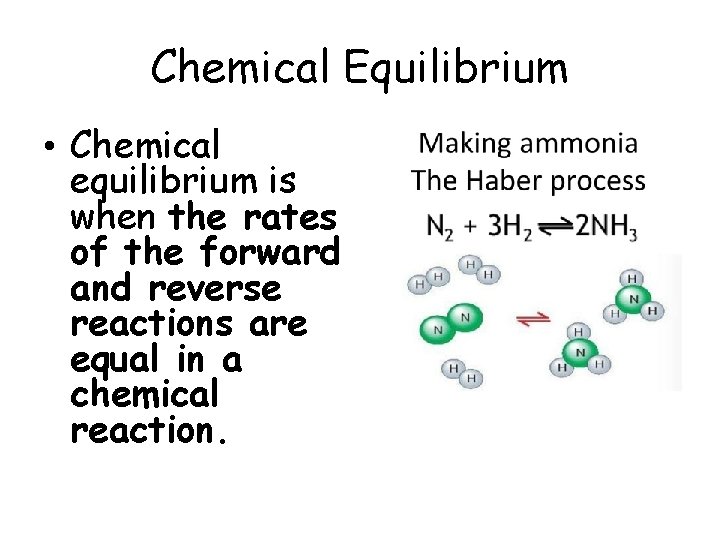
Chemical Equilibrium • Chemical equilibrium is when the rates of the forward and reverse reactions are equal in a chemical reaction.
Le Chatelier’s Principle • Explains how a chemical reaction at equilibrium responds to relieve any stress on that reaction. STRESS MEANS CHANGE • Reaction will counter the stress by shifting to the left to produce more reactant, or shift to the right to produce more product. – Shift means produce more of… • Sources of Stress: A) Changes in Concentration(amount) B) Changes in Temperature(added/subtracted heat) C) Changes in Pressure (only if gases are present)
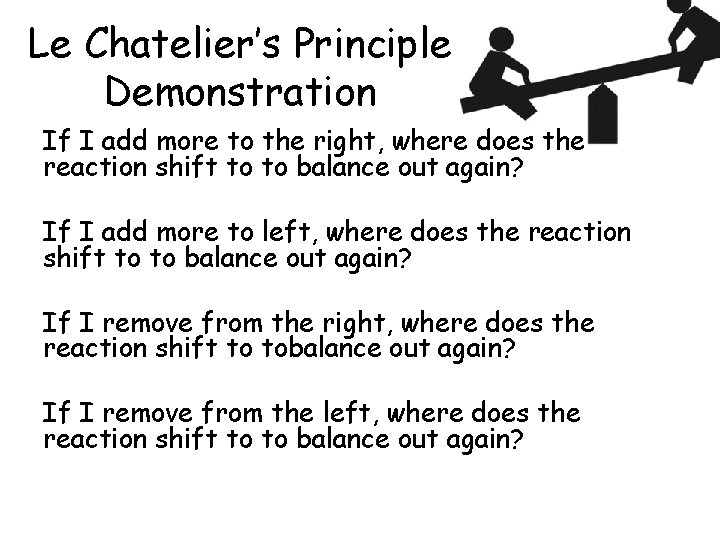
Le Chatelier’s Principle Demonstration If I add more to the right, where does the reaction shift to to balance out again? If I add more to left, where does the reaction shift to to balance out again? If I remove from the right, where does the reaction shift to tobalance out again? If I remove from the left, where does the reaction shift to to balance out again?

How will the reaction shift to counter the addition of more of a substance? • If I add more on one side, the reaction will shift to produce more of the other side – Add to the right (product side), reaction shifts to the left (reactant side) producing more of the reactant and using up the product – Add to the left (reactant side), reaction shifts to the right (product side) producing more of the products and using up the reactants

How will the reaction shift to counter the removal of a substance? • If I remove on one side, the reaction will shift to produce more of the same side – Remove from the right (product side), reaction shifts to the right (product side) producing more of the product and using up the reactant – Remove from the left (reactant side), reaction shifts to the left (reactant side) producing more of the reactants and using up the products
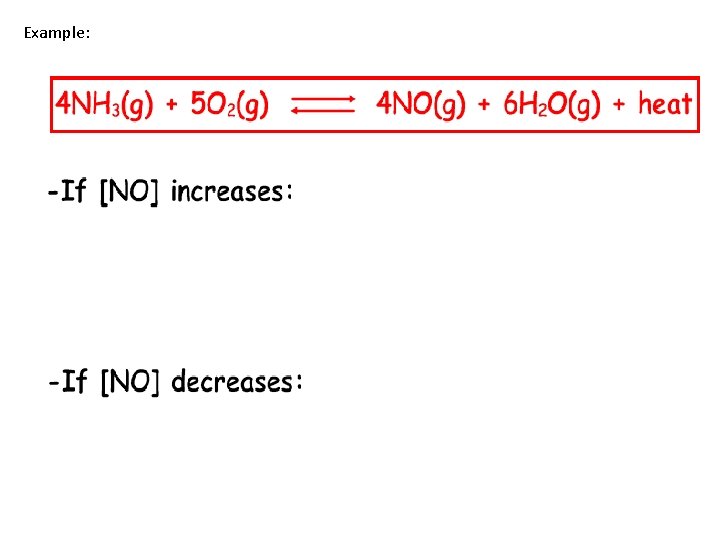
Example:

Question 1 Given the reaction at equilibrium: N 2(g) + O 2(g) 2 NO(g) As the concentration of N 2(g) increases, the concentration of O 2(g) will a. Decrease b. Increase c. Remain the same
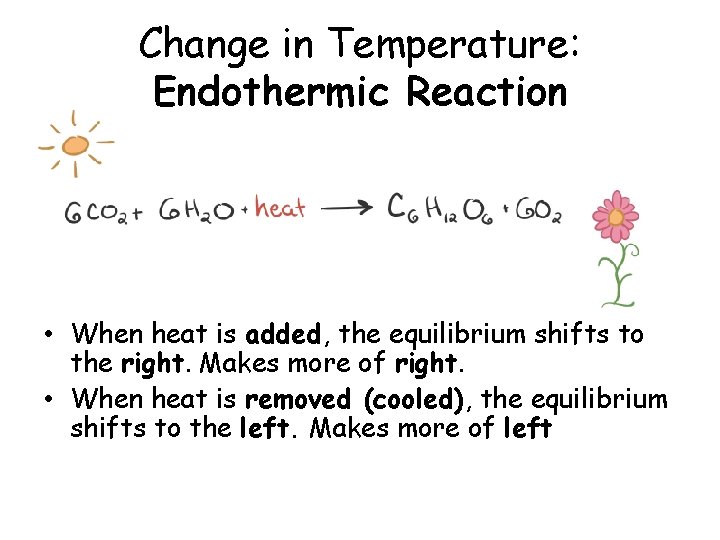
Change in Temperature: Endothermic Reaction • When heat is added, the equilibrium shifts to the right. Makes more of right. • When heat is removed (cooled), the equilibrium shifts to the left. Makes more of left

Change in Temperature: Exothermic Reaction N 2 + 3 H 2 2 NH 3 + 91. 8 k. J • When heat is added, equilibrium shifts to the left. Makes more of left. • When heat is removed (cooled), the equilibrium shifts to the right. Makes more of right.
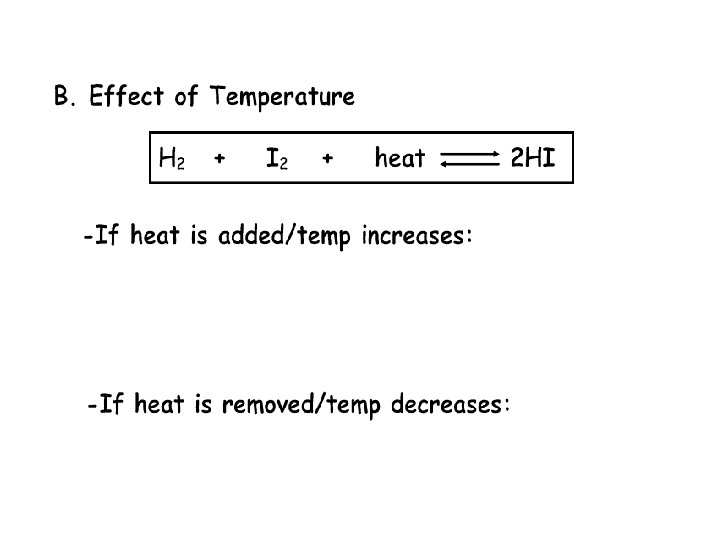

Question 2 Given the equilibrium reaction at constant pressure: 2 HBr(g) + 72. 7 k. J H 2(g) + Br 2(g) When the temperature is increased, the equilibrium will shift to the a. right, and the concentration of HBr(g) will decrease b. Right, and the concentration of HBr(g) will increase c. Left, and the concentration of HBr(g) will decrease d. Left, and the concentration of HBr(g) will increase
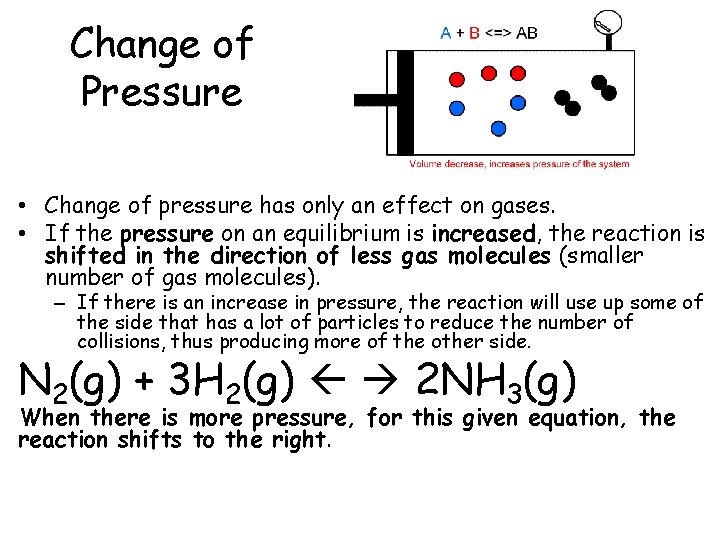
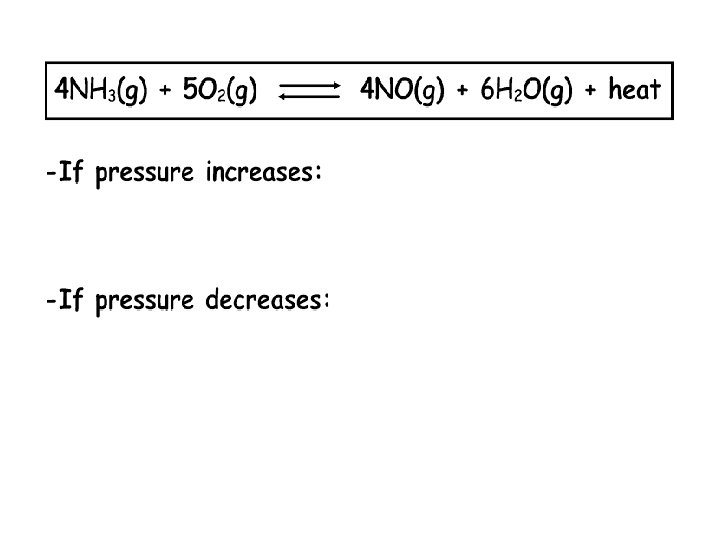
Change of Pressure • Change of pressure has only an effect on gases. • If the pressure on an equilibrium is increased, the reaction is shifted in the direction of less gas molecules (smaller number of gas molecules). – If there is an increase in pressure, the reaction will use up some of the side that has a lot of particles to reduce the number of collisions, thus producing more of the other side. N 2(g) + 3 H 2(g) 2 NH 3(g) When there is more pressure, for this given equation, the reaction shifts to the right.

Question 3 In which reaction will the point of equilibrium shift to the left when the pressure in the system is increased? a. C(s) + O 2(g) CO 2(g) b. Ca. CO 3(s) Ca. O(s) + CO 2(g) c. 2 Mg(s) + O 2(g) 2 Mg. O(s) d. 2 H 2(g) + O 2(g) 2 H 2 O(g)


Summary Add to left, shift to right Add to right, shift to left Remove from left, shift to left Remove from right, shift to right If increase pressure, shift to the side with less gas molecules • The side in which the reaction shifts is the side whose amount increases; and the other side’s amount decreases. • • •
- Slides: 24