Aim What are the five general types of




- Slides: 11

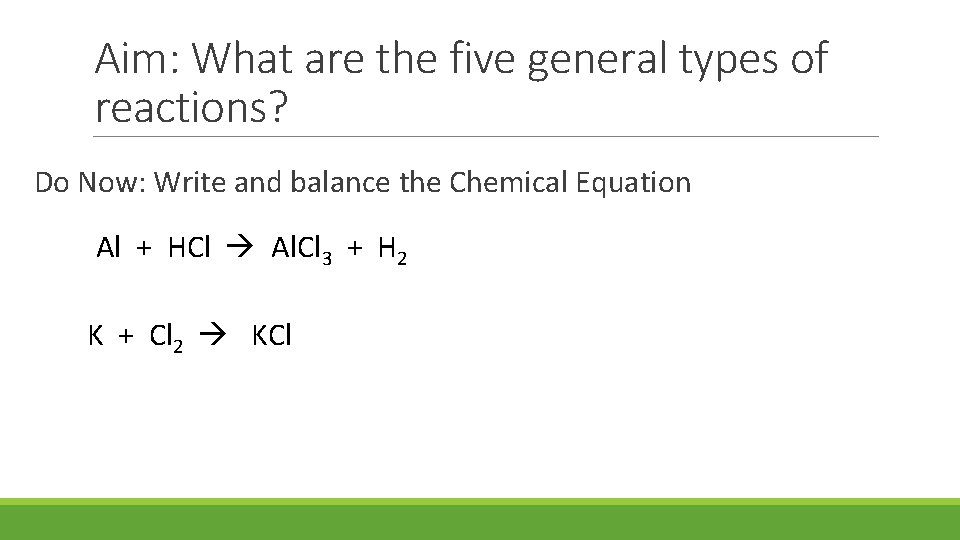
Aim: What are the five general types of reactions? Do Now: Write and balance the Chemical Equation Al + HCl Al. Cl 3 + H 2 K + Cl 2 KCl

Types pf Reactions The four general types of reactions are: §Combination/Synthesis Reactions §Decomposition §Single-Replacement §Double-Replacement

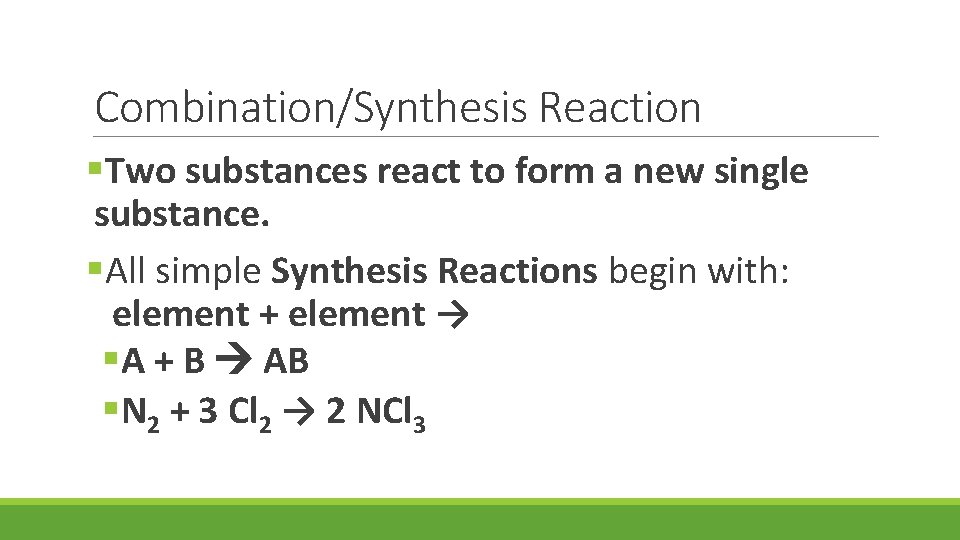
Combination/Synthesis Reaction §Two substances react to form a new single substance. §All simple Synthesis Reactions begin with: element + element → §A + B AB §N 2 + 3 Cl 2 → 2 NCl 3

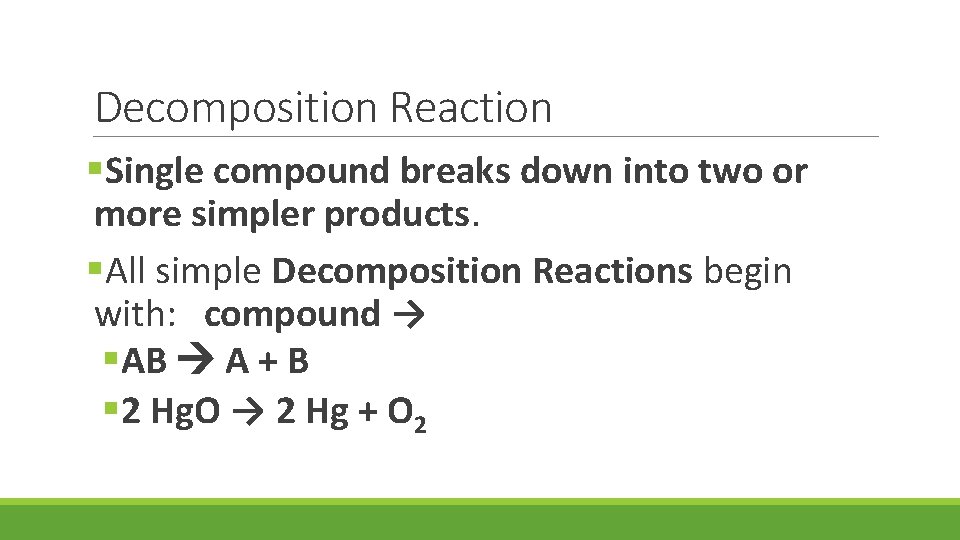
Decomposition Reaction §Single compound breaks down into two or more simpler products. §All simple Decomposition Reactions begin with: compound → §AB A + B § 2 Hg. O → 2 Hg + O 2

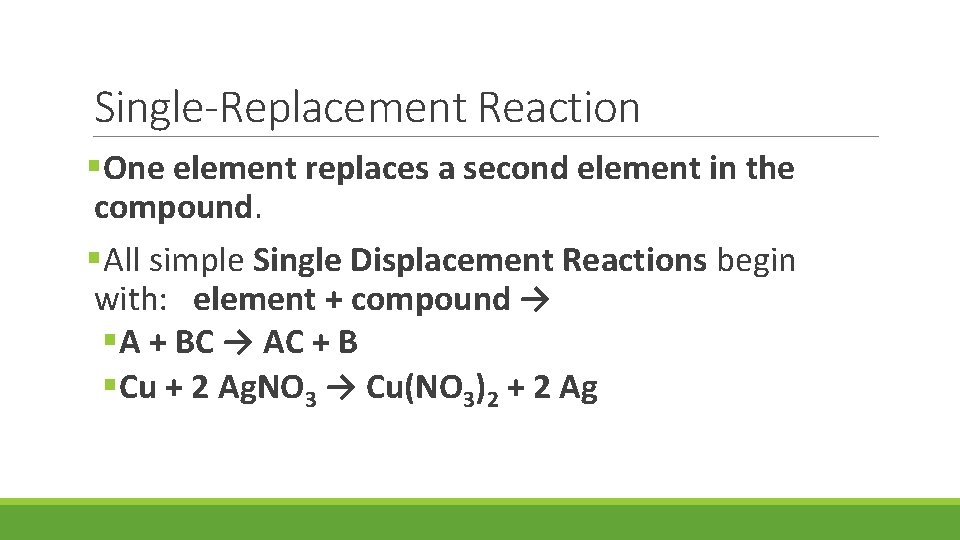
Single-Replacement Reaction §One element replaces a second element in the compound. §All simple Single Displacement Reactions begin with: element + compound → §A + BC → AC + B §Cu + 2 Ag. NO 3 → Cu(NO 3)2 + 2 Ag

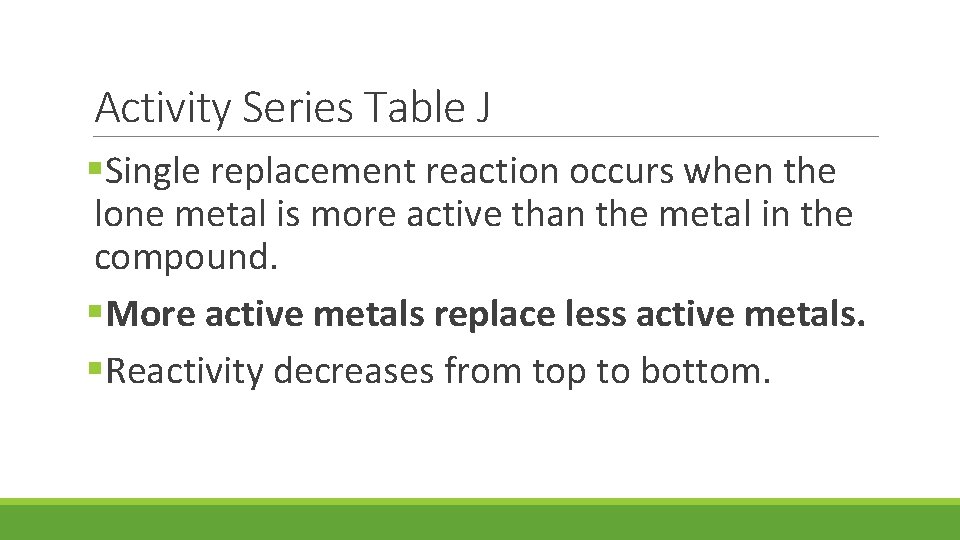
Activity Series Table J §Single replacement reaction occurs when the lone metal is more active than the metal in the compound. §More active metals replace less active metals. §Reactivity decreases from top to bottom.

Using Table J determine which of the following reaction will not occur (no reaction) A) Cl 2 + 2 Na. Br B) Mg + 2 HCl C) Cu + Ag. NO 3 D) Cu + Al. Cl 3 If the element that is to replace the other in the compound is higher on the chart, the reaction will occur. If it is below, there will be no reaction.

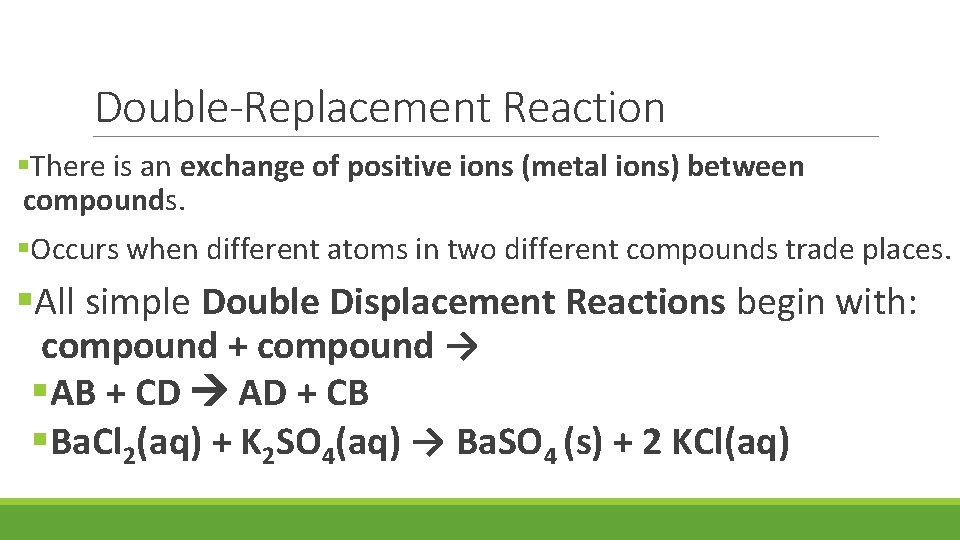
Double-Replacement Reaction §There is an exchange of positive ions (metal ions) between compounds. §Occurs when different atoms in two different compounds trade places. §All simple Double Displacement Reactions begin with: compound + compound → §AB + CD AD + CB §Ba. Cl 2(aq) + K 2 SO 4(aq) → Ba. SO 4 (s) + 2 KCl(aq)

Double-Replacement Reaction Cont. A reaction will occur if one of the products is a solid (s), a gas (g), or a molecular substance e. g. H 2 O, is formed.

Follow this series of questions. When you can answer "yes" to a question, then stop! 1) Does your reaction have oxygen as one of it's reactants and carbon dioxide and water as products? If yes, then it's a combustion reaction 2) Does your reaction have two (or more) chemicals combining to form one chemical? If yes, then it's a synthesis reaction 3) Does your reaction have one large molecule falling apart to make several small ones? If yes, then it's a decomposition reaction 4) Does your reaction have any molecules that contain only one element? If yes, then it's a single displacement reaction 5) If you haven't answered "yes" to any of the questions above, then you've got a double displacement reaction

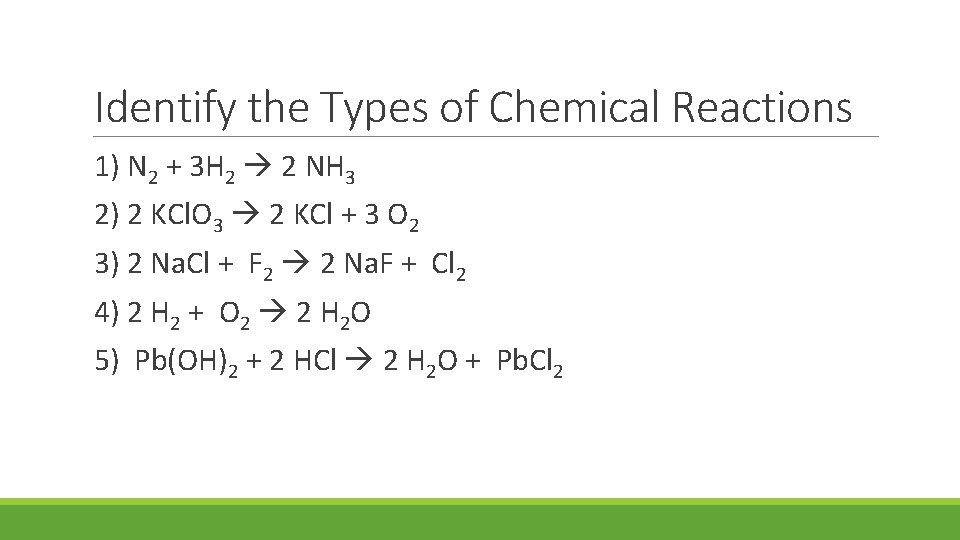
Identify the Types of Chemical Reactions 1) N 2 + 3 H 2 2 NH 3 2) 2 KCl. O 3 2 KCl + 3 O 2 3) 2 Na. Cl + F 2 2 Na. F + Cl 2 4) 2 H 2 + O 2 2 H 2 O 5) Pb(OH)2 + 2 HCl 2 H 2 O + Pb. Cl 2