Aim What are solubility factors Do now Define

Aim: What are solubility factors? Do now: Define what a solution is and list some properties of solutions.

• Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a liquid solvent to form a homogeneous solution of the solute in the solvent.

• The solubility of a substance fundamentally depends on the used solvent as well as on temperature and pressure.

• The extent of solubility ranges widely, from infinitely soluble (fully miscible) such as ethanol in water, to poorly soluble, such as silver chloride in water. • The term insoluble is often applied to poorly or very poorly soluble compounds.
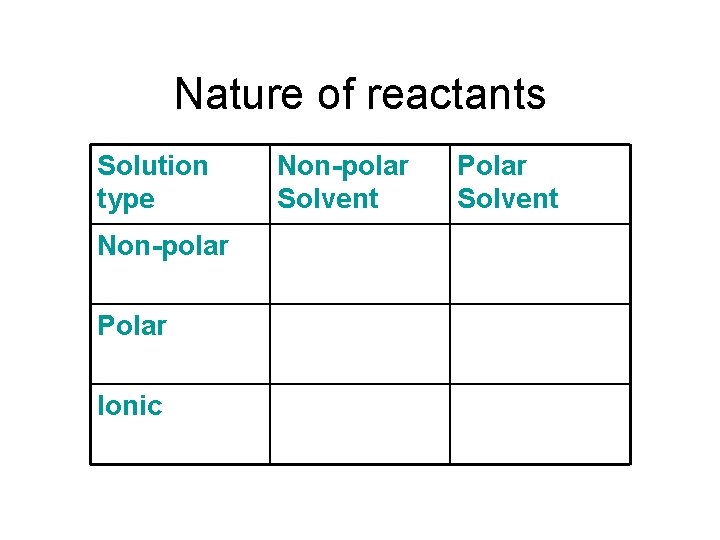
Nature of reactants Solution type Non-polar Polar Ionic Non-polar Solvent Polar Solvent

Like dissolves Like.

Temperature • As temperature increases solids become more soluble in water • As temperature increases gasses become less soluble in water.

Pressure • Pressure has little or no effect on the solubility of solid or liquid solutes. • As pressure increases, the solubility of gasses in liquids increases.
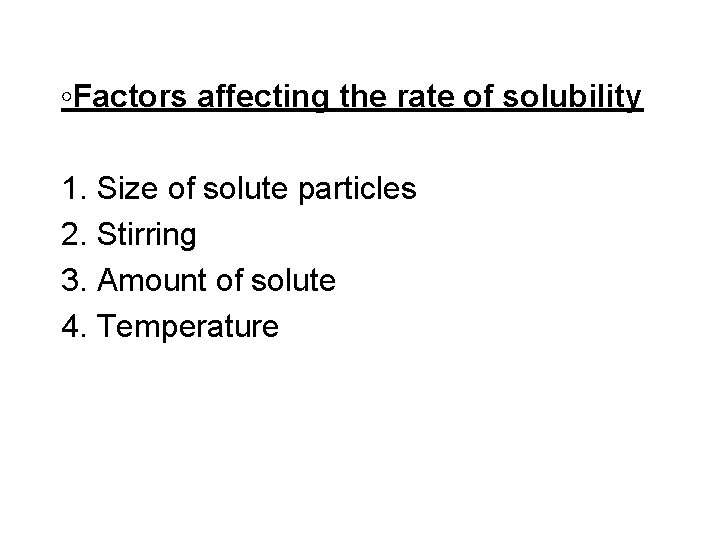
◦Factors affecting the rate of solubility 1. Size of solute particles 2. Stirring 3. Amount of solute 4. Temperature
HOW DO WE READ SOLUBILITY GRAPHS
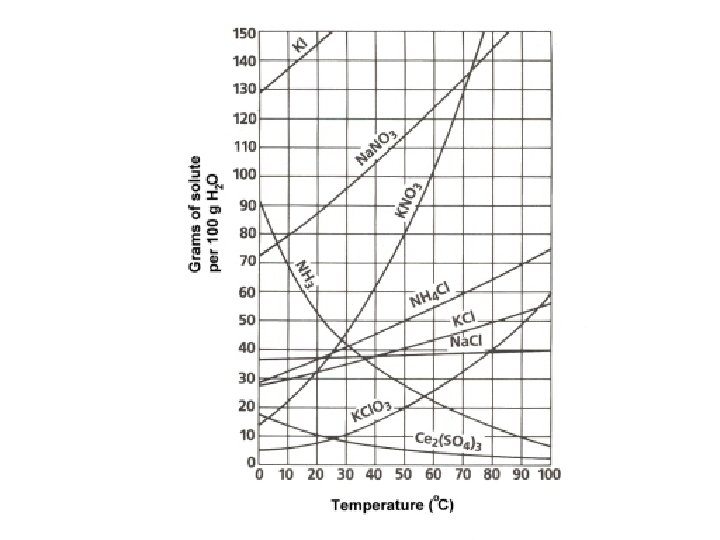
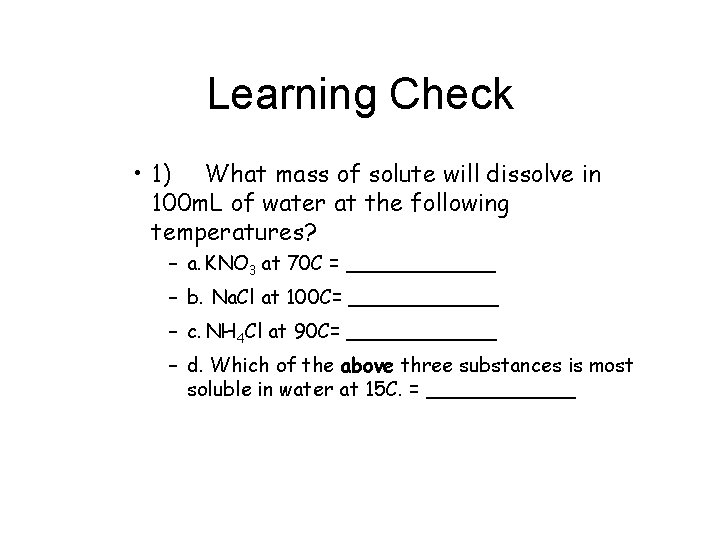
Learning Check • 1) What mass of solute will dissolve in 100 m. L of water at the following temperatures? – a. KNO 3 at 70 C = ______ – b. Na. Cl at 100 C= ______ – c. NH 4 Cl at 90 C= ______ – d. Which of the above three substances is most soluble in water at 15 C. = ______
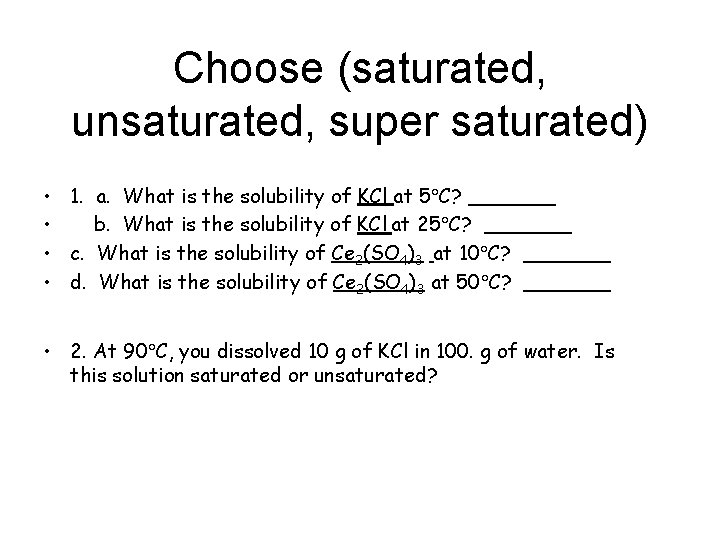
Choose (saturated, unsaturated, super saturated) • 1. a. What is the solubility of KCl at 5 C? _______ • b. What is the solubility of KCl at 25 C? _______ • c. What is the solubility of Ce 2(SO 4)3 at 10 C? _______ • d. What is the solubility of Ce 2(SO 4)3 at 50 C? _______ • 2. At 90 C, you dissolved 10 g of KCl in 100. g of water. Is this solution saturated or unsaturated?
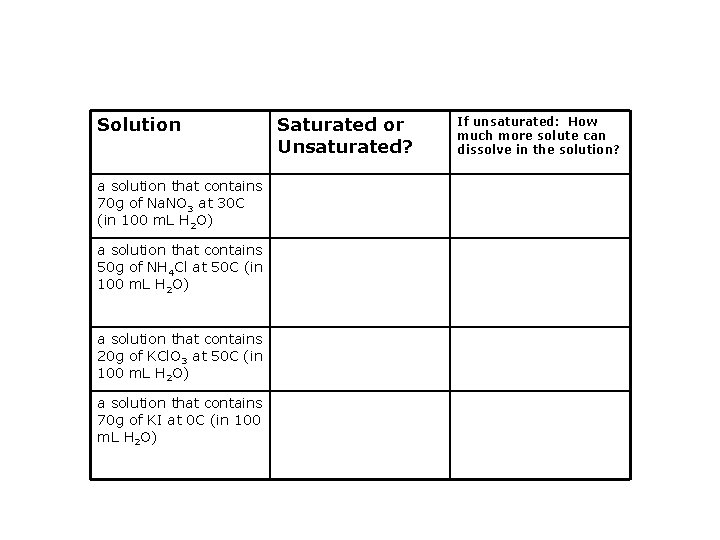
Solution a solution that contains 70 g of Na. NO 3 at 30 C (in 100 m. L H 2 O) a solution that contains 50 g of NH 4 Cl at 50 C (in 100 m. L H 2 O) a solution that contains 20 g of KCl. O 3 at 50 C (in 100 m. L H 2 O) a solution that contains 70 g of KI at 0 C (in 100 m. L H 2 O) Saturated or Unsaturated? If unsaturated: How much more solute can dissolve in the solution?
- Slides: 14