Aim What are molecular compounds Do Now Take



- Slides: 11

Aim: What are molecular compounds? Do Now: Take a copy of the writing assignment. Read through the writing assignment. Due 11/18

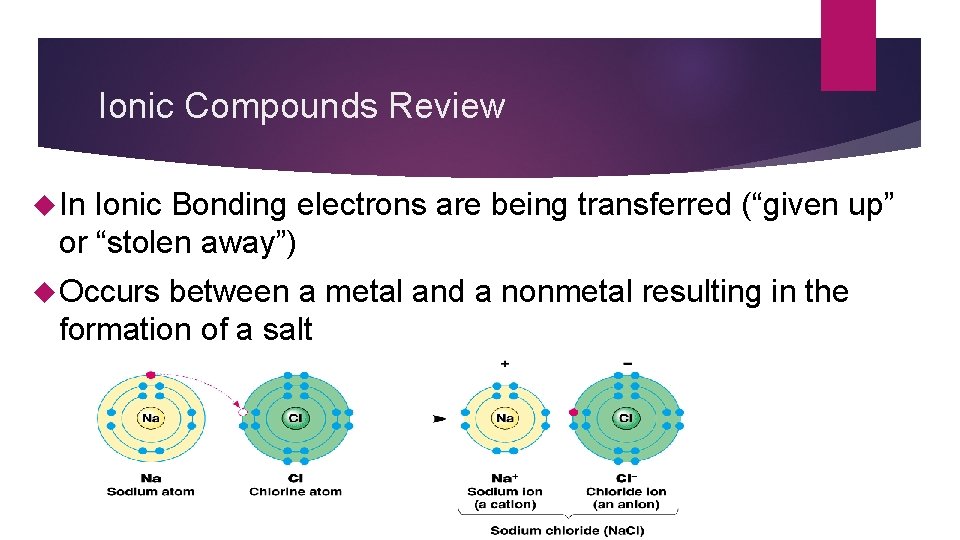
Ionic Compounds Review In Ionic Bonding electrons are being transferred (“given up” or “stolen away”) Occurs between a metal and a nonmetal resulting in the formation of a salt

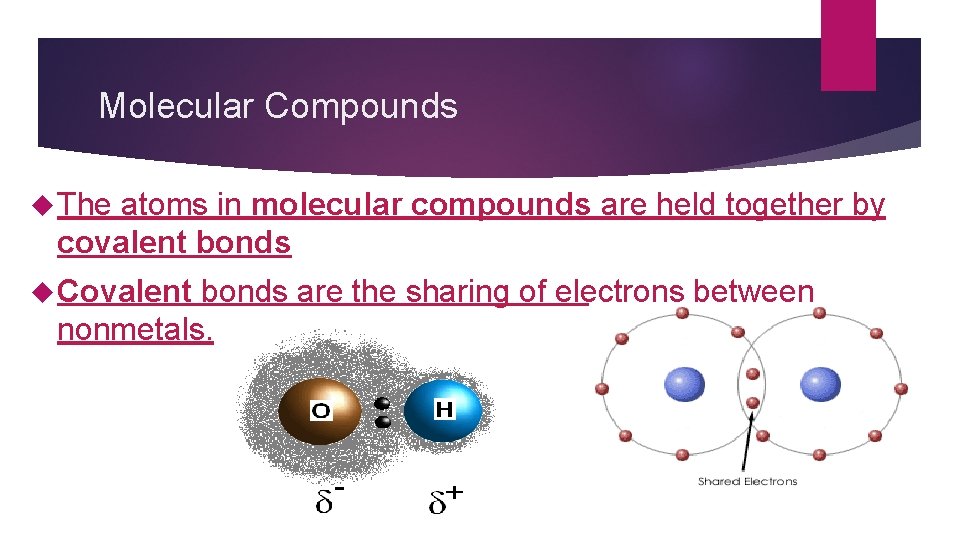
Molecular Compounds The atoms in molecular compounds are held together by covalent bonds Covalent bonds are the sharing of electrons between nonmetals.

Molecule A molecule is a neutral group of atoms joined together by covalent bonds Diatomic molecules- two atomscovalently bonded to each other Ex: HCl H 2, O 2, N 2, Cl 2, Br 2, I 2, F 2

Salt vs Molecules A salt is a metal cation and a nonmetal anion joined together by an ionic bond A molecule is a group of atoms joined together by a covalent bond A compound is a group of two or more elements bonded together (ionic or covalent)

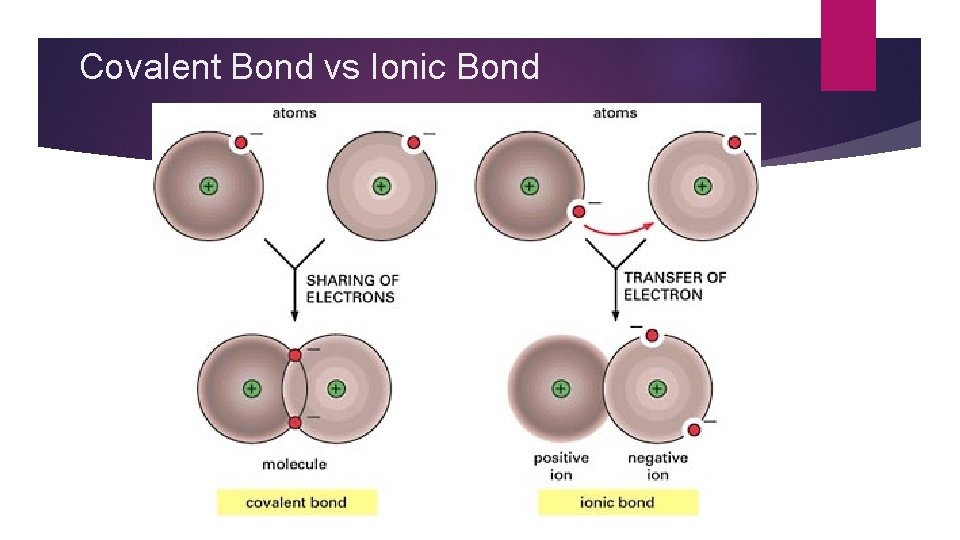
Covalent Bond vs Ionic Bond

Properties of Molecular Compounds Mostly Lower liquid or gases at room temperature melting points than ionic compounds (meaning the bonds are weaker than ionic bonds)

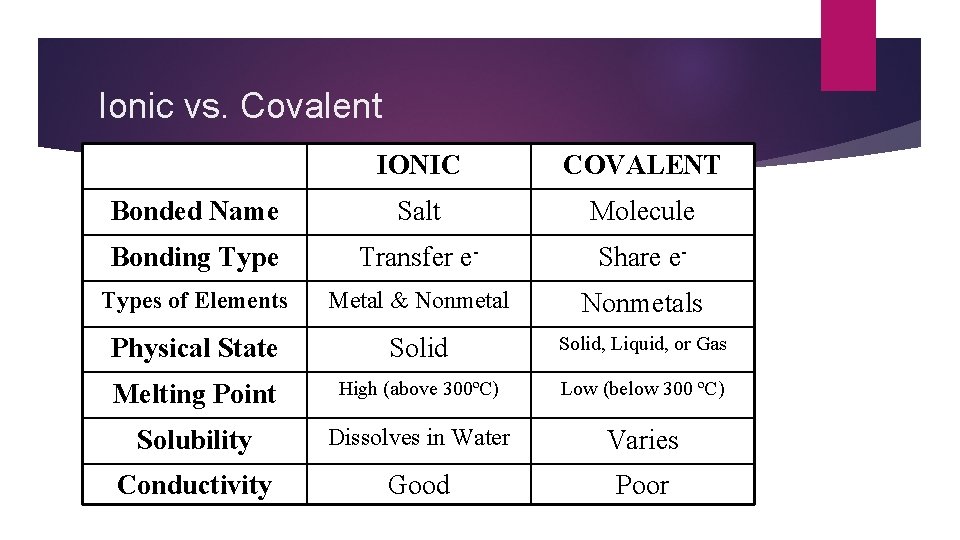
Ionic vs. Covalent IONIC COVALENT Bonded Name Salt Molecule Bonding Type Transfer e- Share e- Types of Elements Metal & Nonmetals Physical State Solid, Liquid, or Gas Melting Point High (above 300ºC) Low (below 300 ºC) Solubility Dissolves in Water Varies Conductivity Good Poor

Molecular Formulas The molecular formula is the formula of a molecular compound It shows how many atoms of each element a molecule contains Ex: H 2 O contains 3 atoms (2 atoms of H, 1 atom of O) C 2 H 6 contains 8 atoms (2 atoms of C, 6 atoms of H)

Practice How many atoms in total and of each do the following molecular compounds contain? 1. H 2 2. CO 3. CO 2 4. NH 3 5. C 2 H 6 O

Practice: True or False 1. 2. 3. 4. 5. All molecular compounds are composed of atoms of two or more elements. All compounds are molecules. Molecular compounds are composed of two or more nonmetals. Atoms in molecular compounds exchange electrons. Molecular compounds have higher melting and boiling points than ionic compounds.