Aim What are covalent bonds and how are

Aim: What are covalent bonds and how are they formed? �Do Now: explain in terms of electronegativity why you think in covalent bonding there is a sharing of electrons and not a transfer of electrons
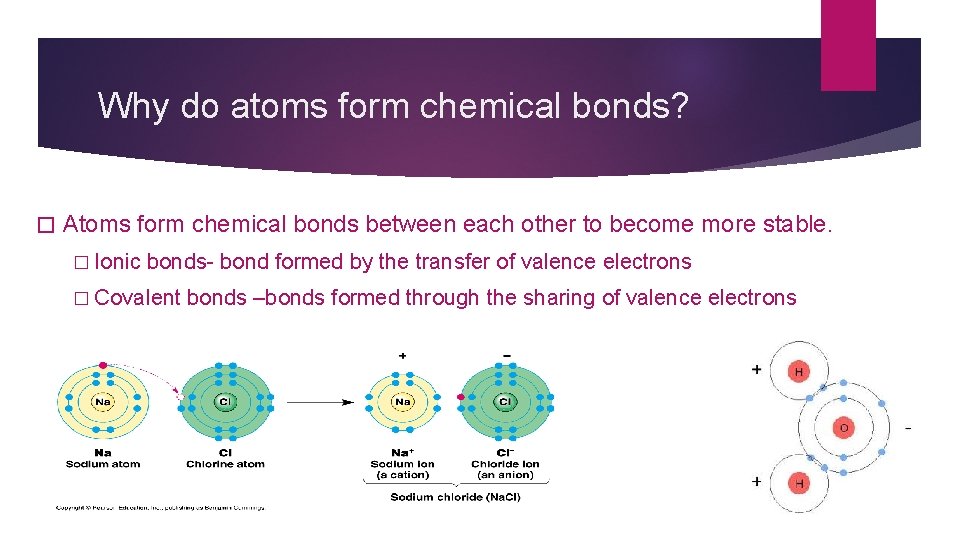
Why do atoms form chemical bonds? � Atoms form chemical bonds between each other to become more stable. � Ionic bonds- bond formed by the transfer of valence electrons � Covalent bonds –bonds formed through the sharing of valence electrons

Chemical Bonds and Energy � A chemical bond has stored energy � When a bond is broken energy is absorbed (taken in); energy is needed to break the bond � When a bond is formed, energy is released, and the compounds formed have lower energy
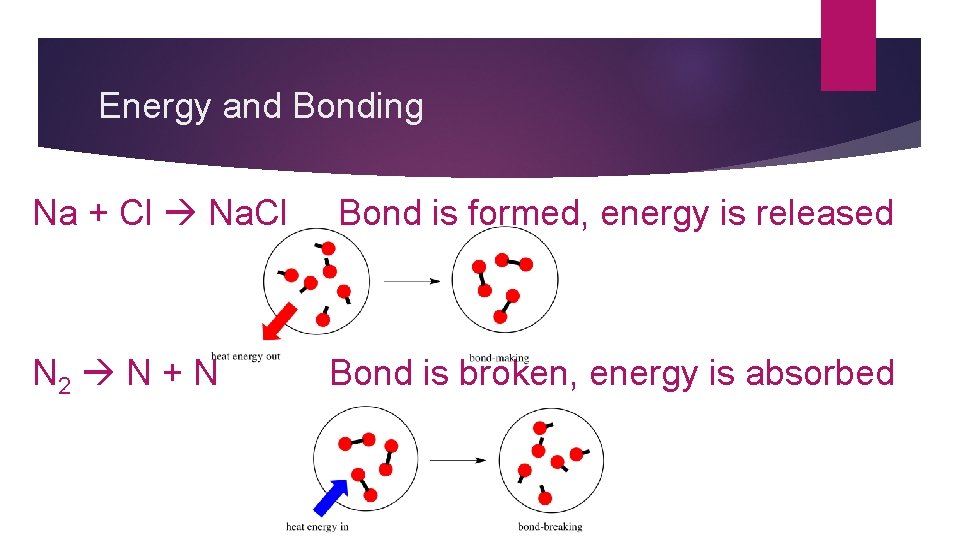
Energy and Bonding Na + Cl Na. Cl Bond is formed, energy is released N 2 N + N Bond is broken, energy is absorbed
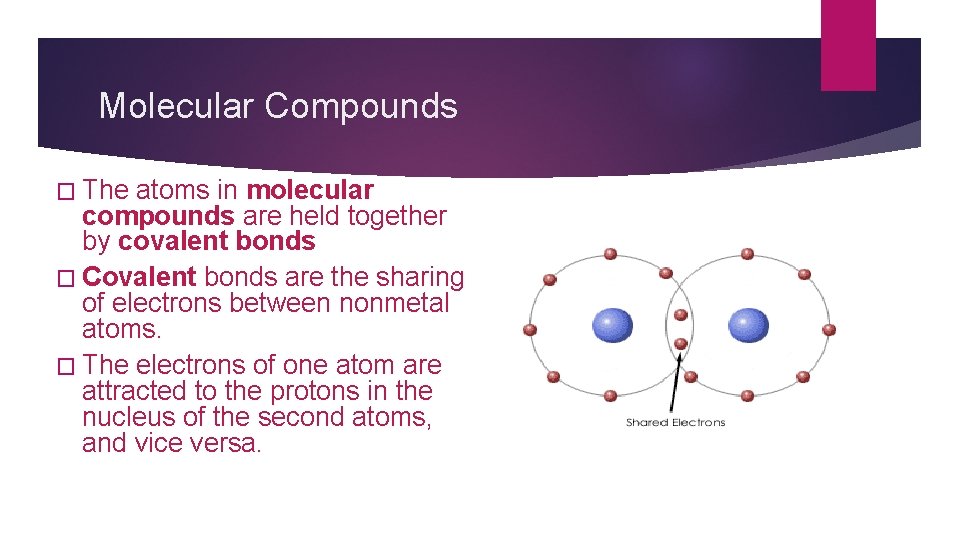
Molecular Compounds � The atoms in molecular compounds are held together by covalent bonds � Covalent bonds are the sharing of electrons between nonmetal atoms. � The electrons of one atom are attracted to the protons in the nucleus of the second atoms, and vice versa.
Molecule �A molecule is a neutral group of atoms joined together by covalent bonds � Diatomic molecules- two atoms covalently bonded to each other �Examples Br 2, I 2, F 2 �Example of molecules of elements: H 2, O 2, N 2, Cl 2, of molecule of compound: HCl
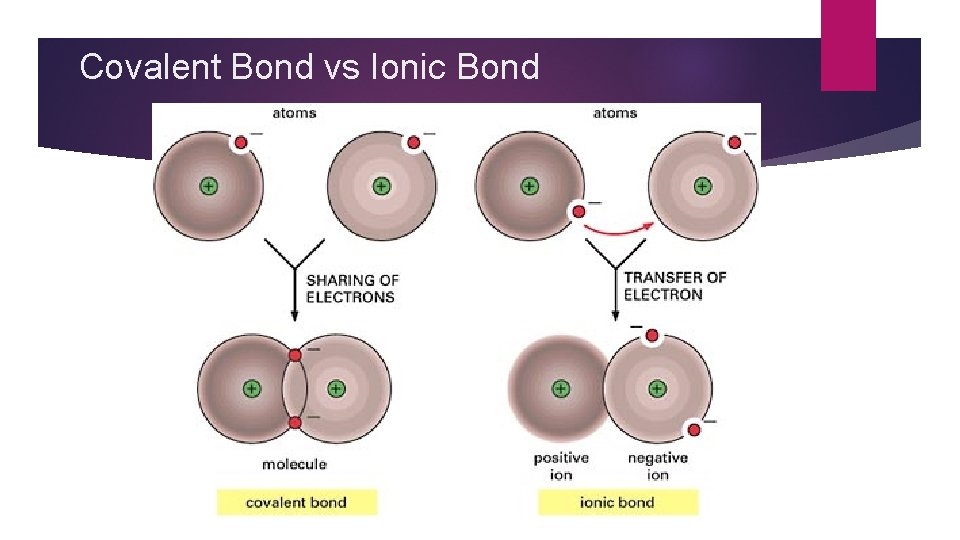
Covalent Bond vs Ionic Bond

In pairs: � Come up with an analogy and write it down describing ionic bonding and covalent bonding. � Examples of analogies: • Bacterial chromosomes are like spaghetti. • Blood vessels are like highways.
Properties of Molecular Compounds � Mostly � Lower liquid or gases at room temperature melting points than ionic compounds (meaning the bonds are weaker than ionic bonds)
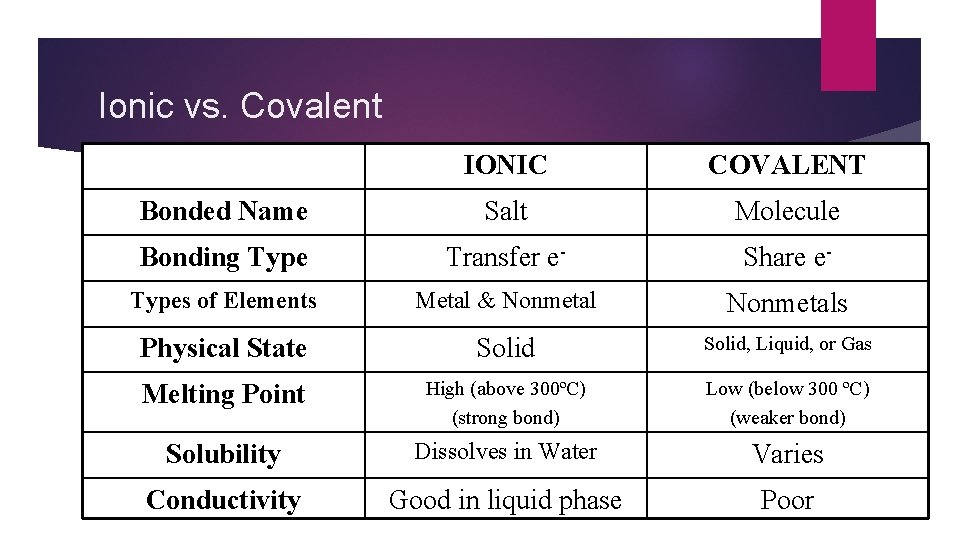
Ionic vs. Covalent IONIC COVALENT Bonded Name Salt Molecule Bonding Type Transfer e- Share e- Types of Elements Metal & Nonmetals Physical State Solid, Liquid, or Gas Melting Point High (above 300ºC) (strong bond) Low (below 300 ºC) (weaker bond) Solubility Dissolves in Water Varies Conductivity Good in liquid phase Poor

Summary � https: //www. ck 12. org/assessment/tools/geometrytool/plix. html? e. Id=SCI. CHE. 404&question. Id=54402 a 515 aa 41377 f 01 caf 54&artifac t. ID=1872630&concept. Collection. Handle=chemistry-: : -covalentbond&collection. Creator. ID=3&back. Url=https%3 A//www. ck 12. org/c/chemistry/cova lent-bond/%3 Fdifficulty%3 Dall%26 by%3 Dck 12%23 interactive&plix_redirect=1
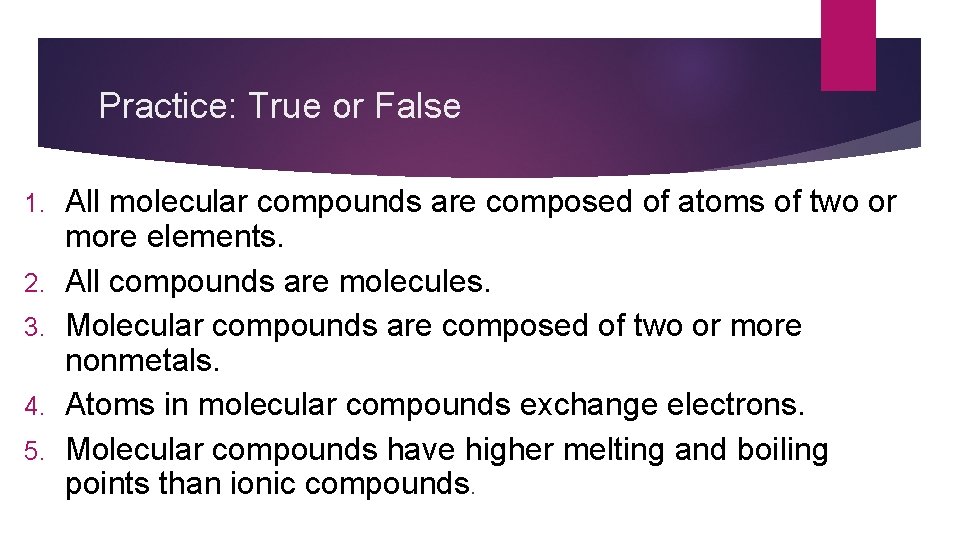
Practice: True or False 1. 2. 3. 4. 5. All molecular compounds are composed of atoms of two or more elements. All compounds are molecules. Molecular compounds are composed of two or more nonmetals. Atoms in molecular compounds exchange electrons. Molecular compounds have higher melting and boiling points than ionic compounds.

Structural Formula �A structural formula is a formula that shows the arrangement of atoms in the molecule of a compound.
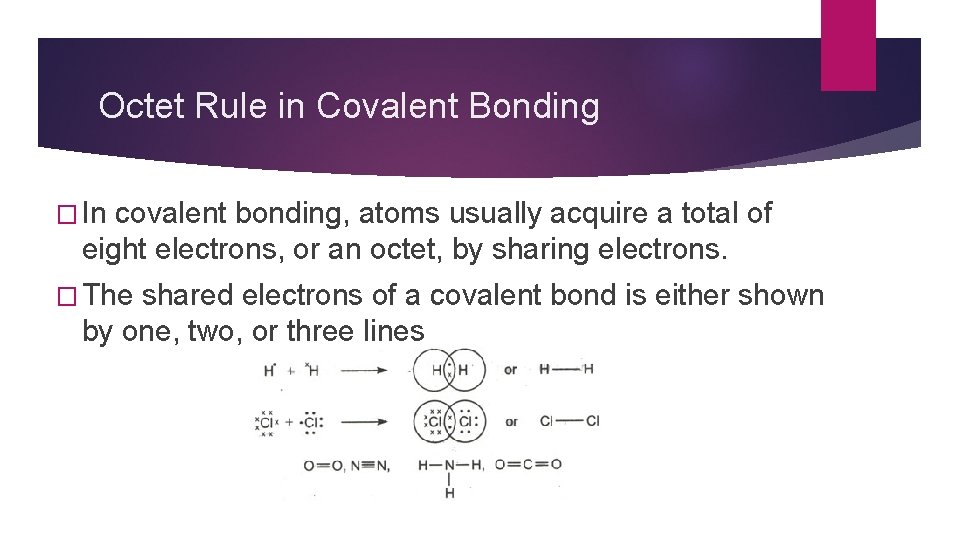
Octet Rule in Covalent Bonding � In covalent bonding, atoms usually acquire a total of eight electrons, or an octet, by sharing electrons. � The shared electrons of a covalent bond is either shown by one, two, or three lines

How can you tell how many bonds an atom can form? � The number of unpaired valence electrons determines how many bonds an atom can form. � Determine how many bonds each of the following atoms can form � Hydrogen � Carbon � Nitrogen � Oxygen � Fluorine
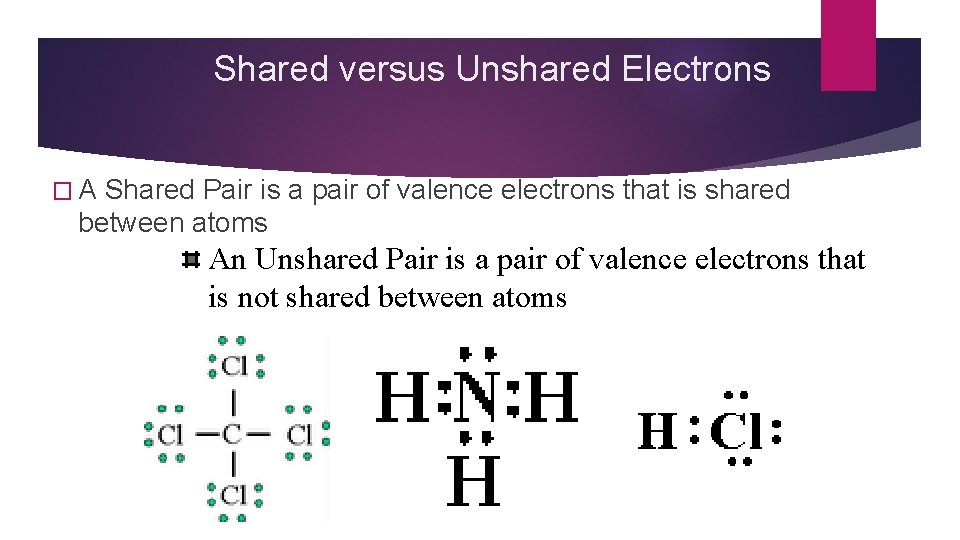
Shared versus Unshared Electrons �A Shared Pair is a pair of valence electrons that is shared between atoms An Unshared Pair is a pair of valence electrons that is not shared between atoms

Single Covalent Bond �A Single Covalent Bond consists of two atoms held together by sharing 1 pair of electrons (2 e-)
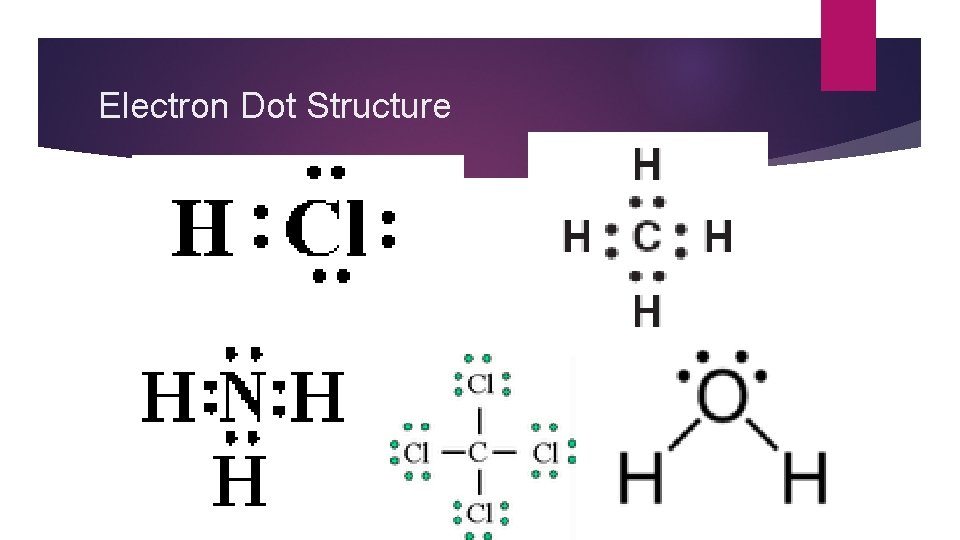
Electron Dot Structure
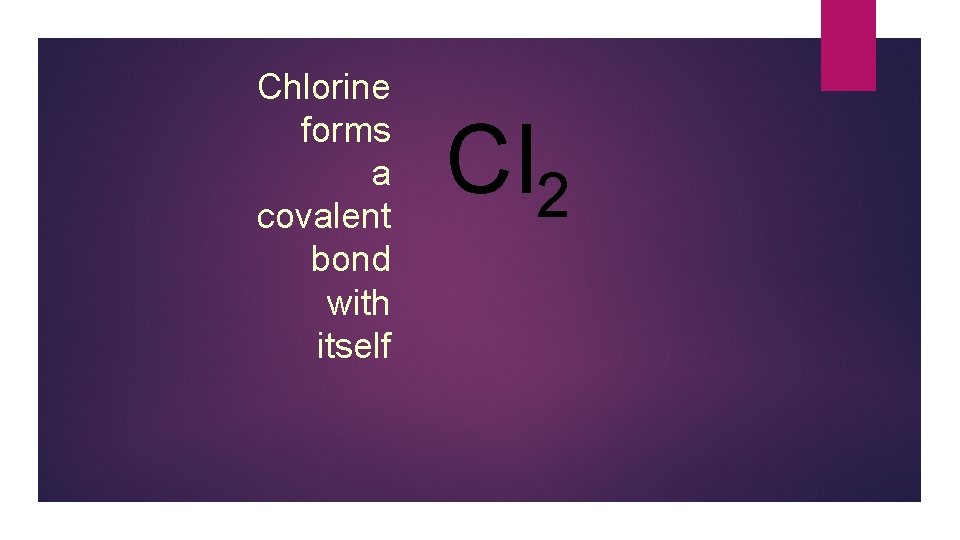
Chlorine forms a covalent bond with itself Cl 2
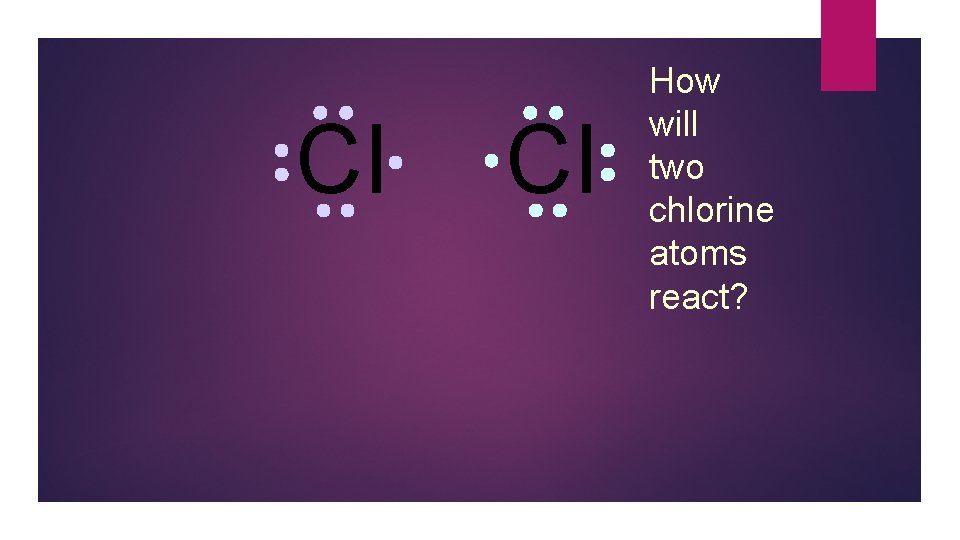
Cl Cl How will two chlorine atoms react?
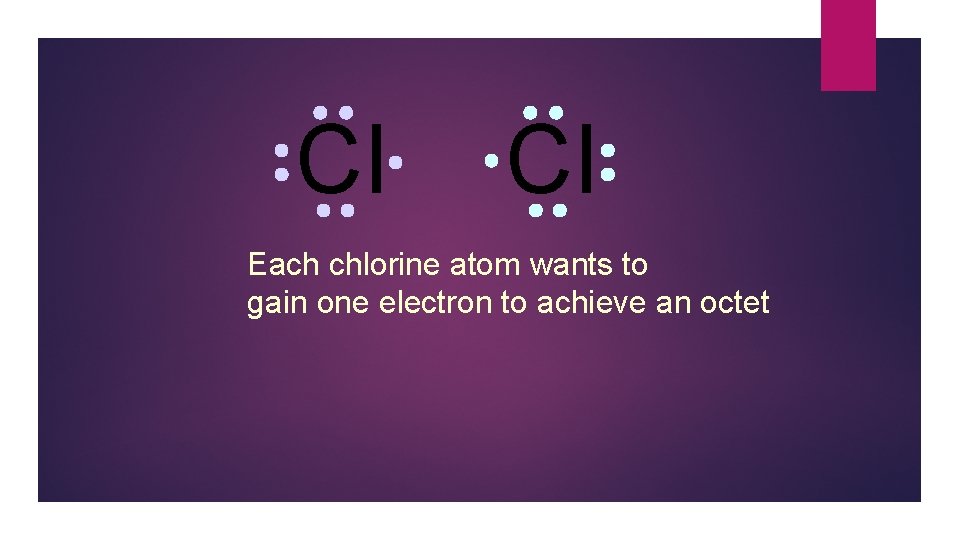
Cl Cl Each chlorine atom wants to gain one electron to achieve an octet
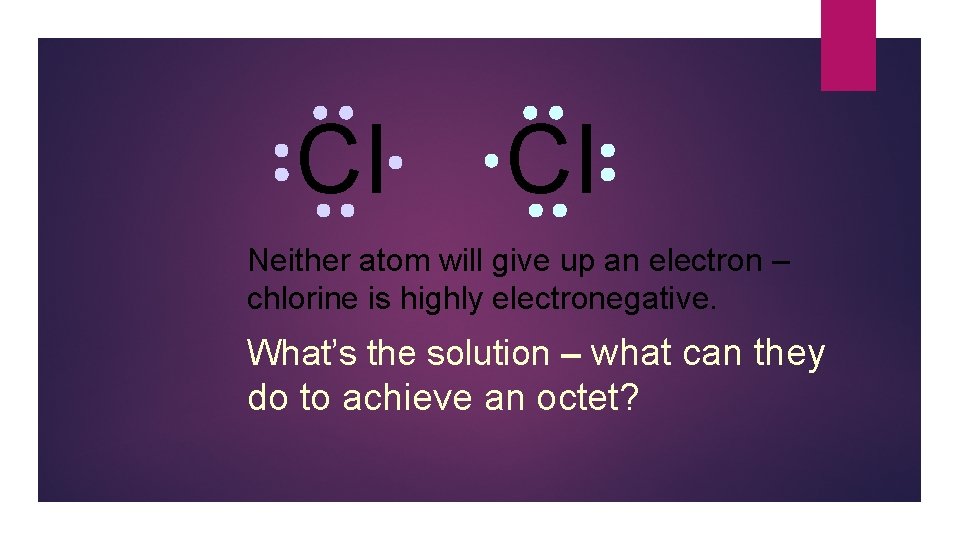
Cl Cl Neither atom will give up an electron – chlorine is highly electronegative. What’s the solution – what can they do to achieve an octet?
Cl Cl
Cl Cl
Cl Cl
Cl Cl
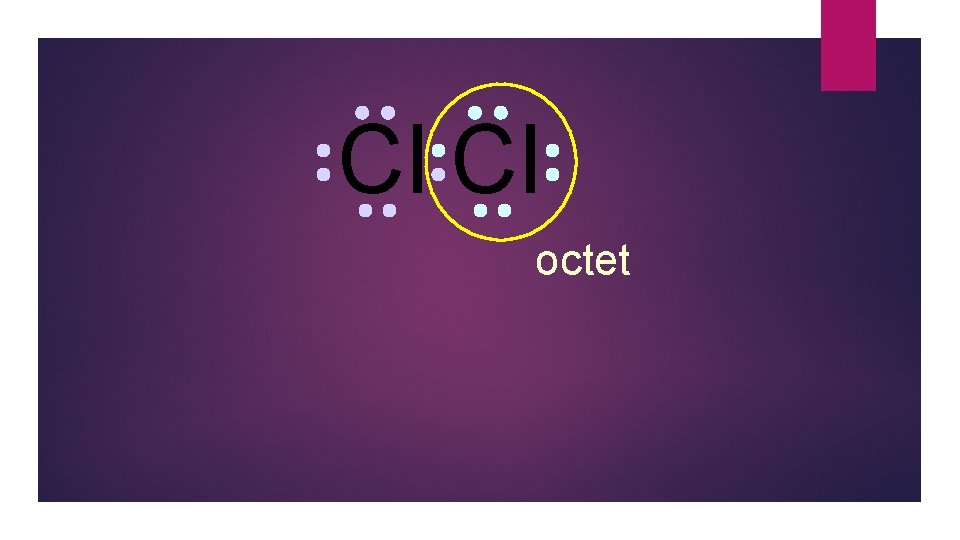
Cl Cl octet
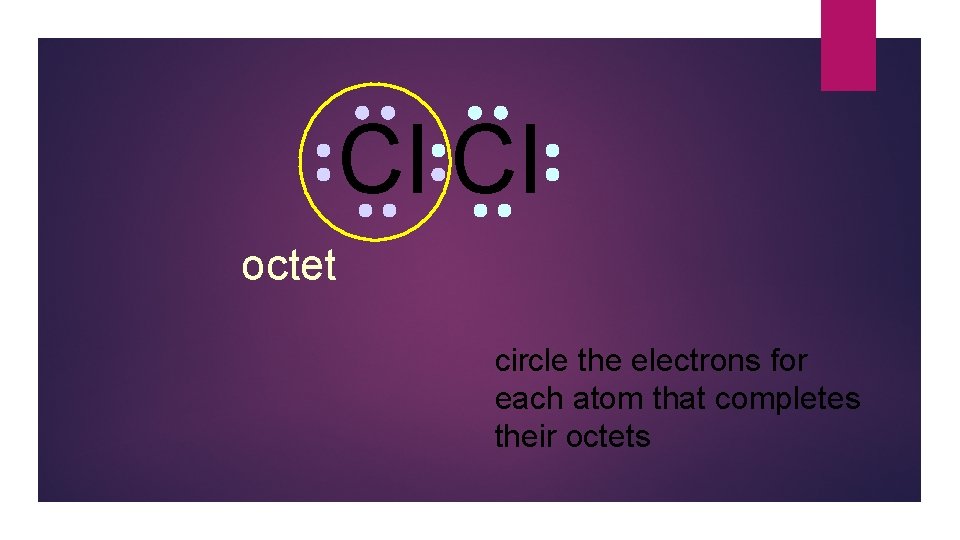
Cl Cl octet circle the electrons for each atom that completes their octets
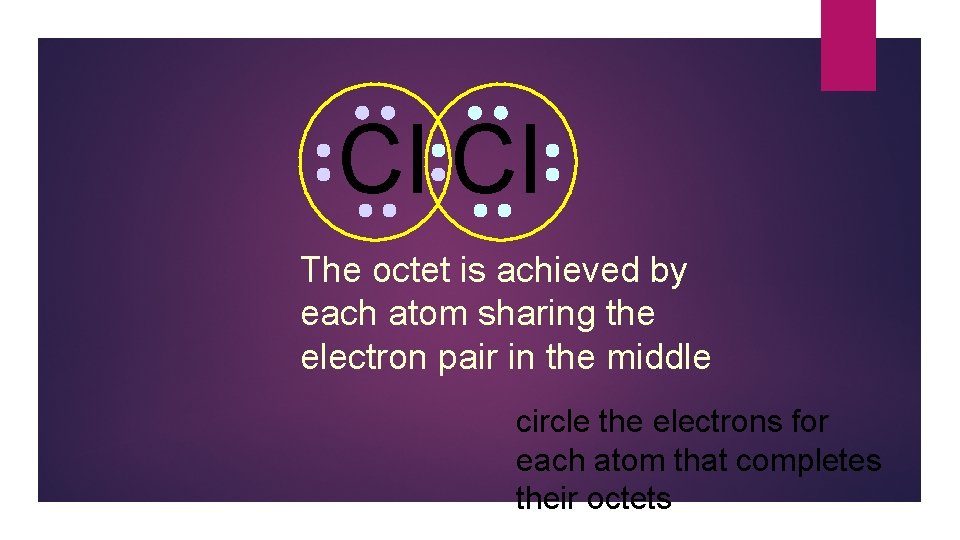
Cl Cl The octet is achieved by each atom sharing the electron pair in the middle circle the electrons for each atom that completes their octets
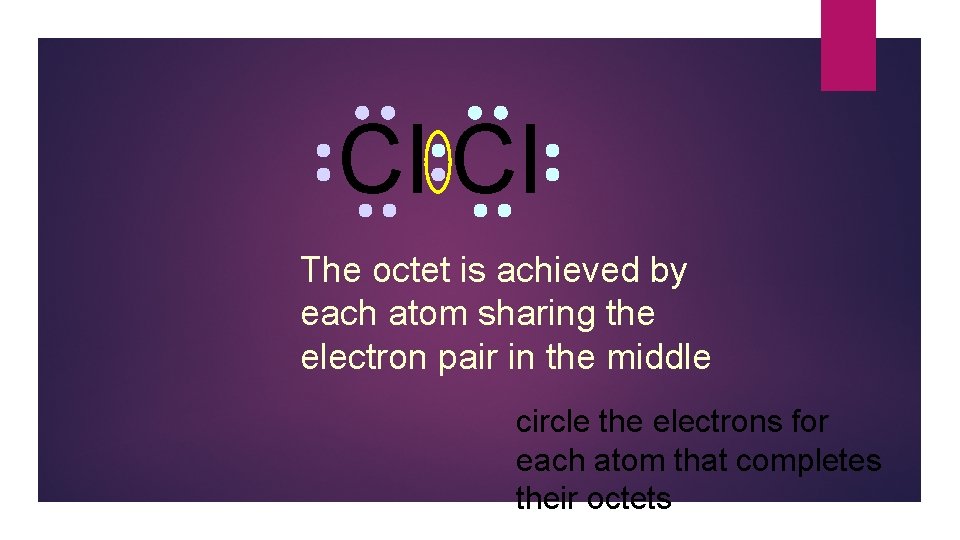
Cl Cl The octet is achieved by each atom sharing the electron pair in the middle circle the electrons for each atom that completes their octets
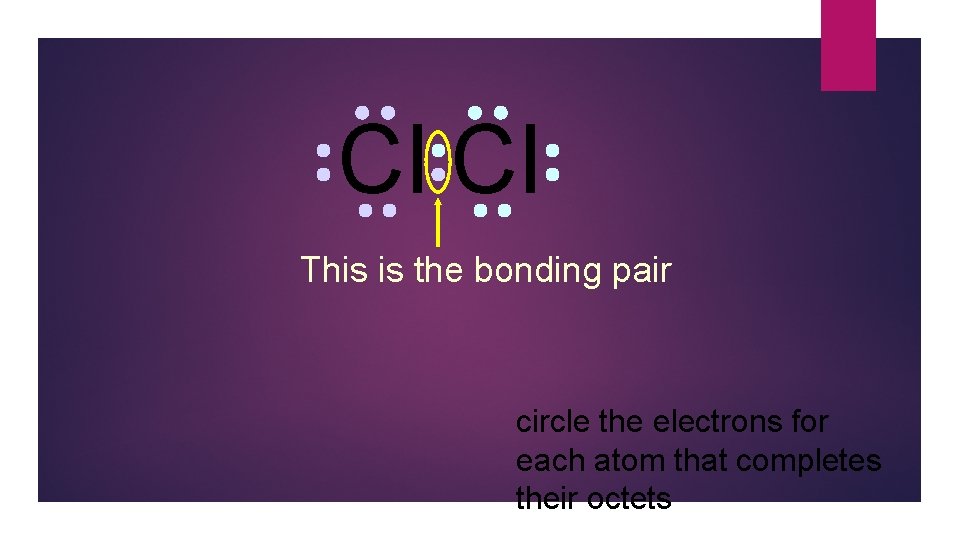
Cl Cl This is the bonding pair circle the electrons for each atom that completes their octets
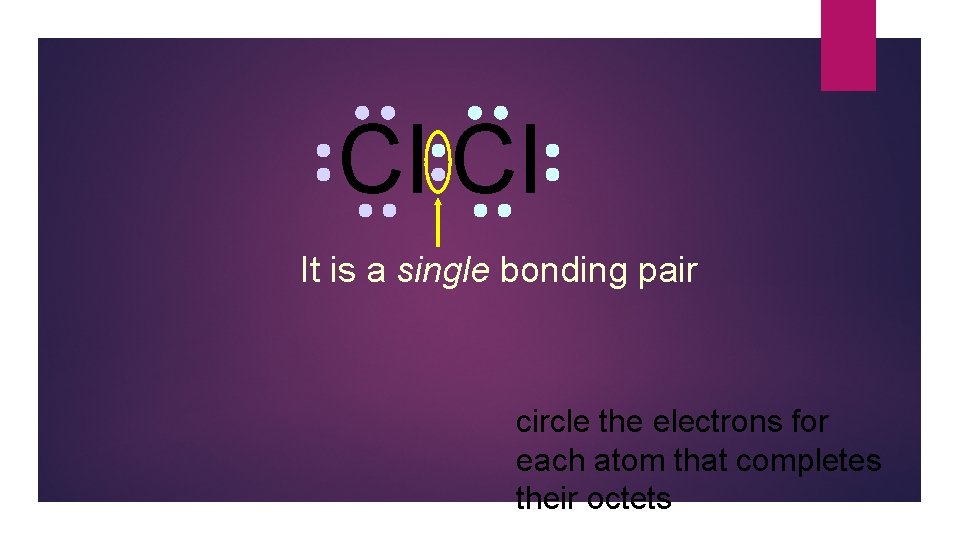
Cl Cl It is a single bonding pair circle the electrons for each atom that completes their octets
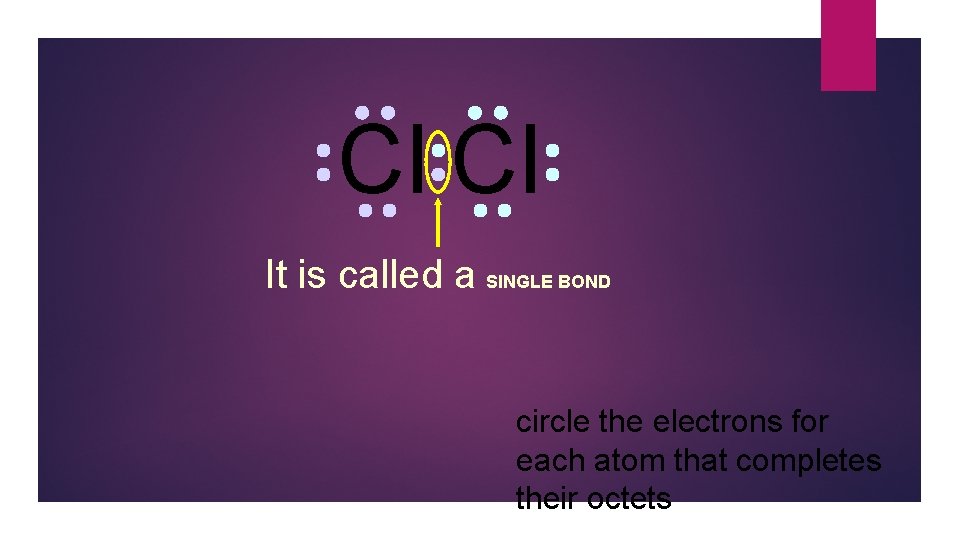
Cl Cl It is called a SINGLE BOND circle the electrons for each atom that completes their octets

Cl Cl Single bonds are abbreviated with a dash circle the electrons for each atom that completes their octets

Cl Cl This is the chlorine molecule, Cl 2 circle the electrons for each atom that completes their octets
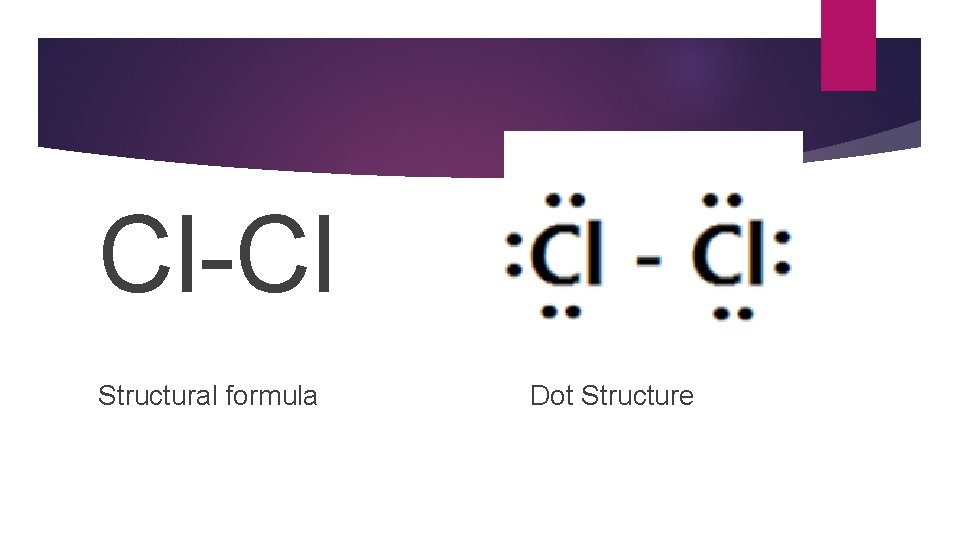
Cl-Cl Structural formula Dot Structure
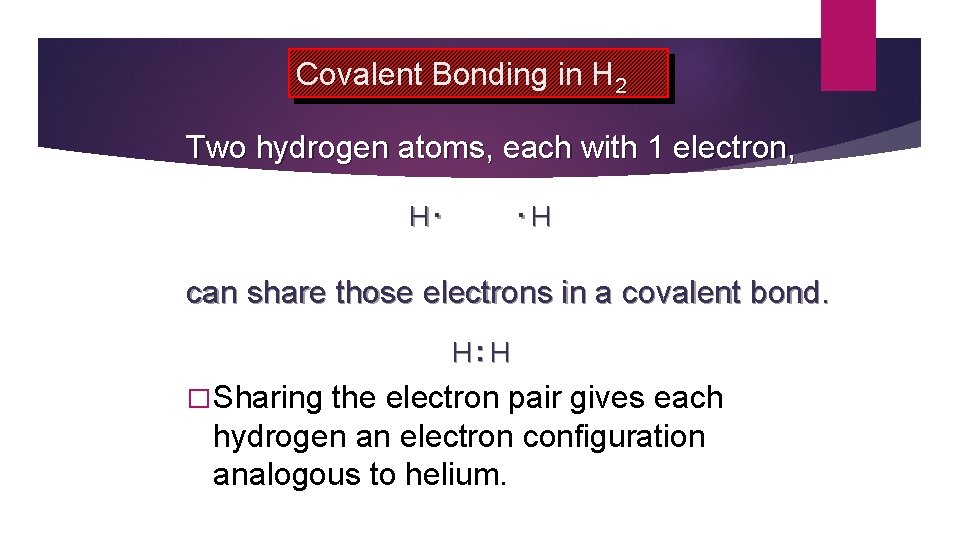
Covalent Bonding in H 2 Two hydrogen atoms, each with 1 electron, H. . H can share those electrons in a covalent bond. H: H � Sharing the electron pair gives each hydrogen an electron configuration analogous to helium.
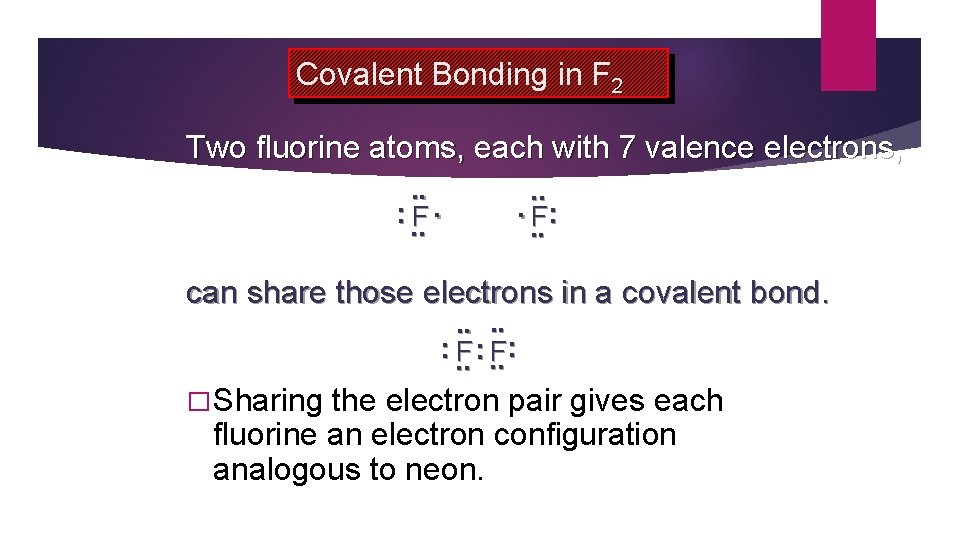
Covalent Bonding in F 2 Two fluorine atoms, each with 7 valence electrons, . . : . . F: . . can share those electrons in a covalent bond. . . : . . F: � Sharing the electron pair gives each fluorine an electron configuration analogous to neon.

The Octet Rule In forming compounds, atoms gain, lose, or share electrons to give a stable electron configuration characterized by 8 valence electrons. . . : . . F: � The octet rule is the most useful in cases involving covalent bonds to C, N, O, and F.
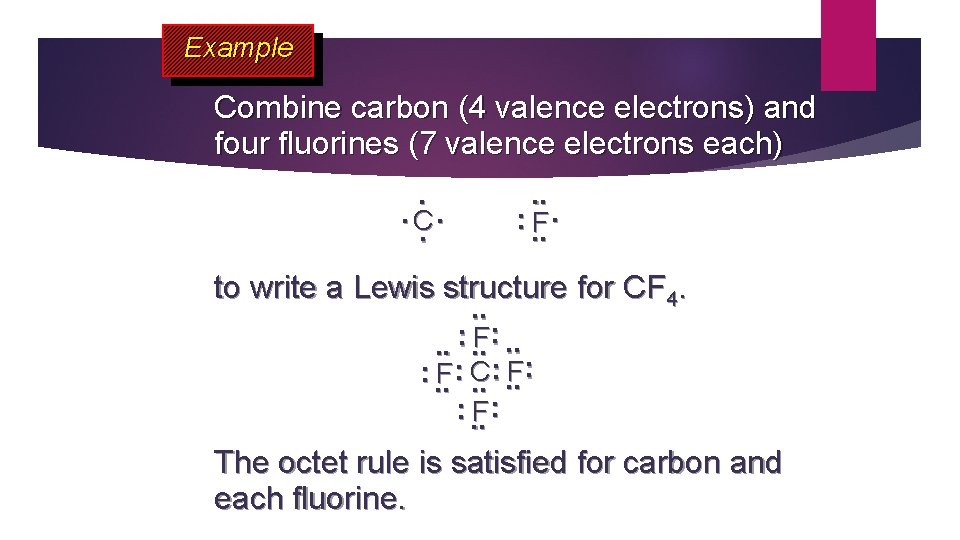
Example Combine carbon (4 valence electrons) and four fluorines (7 valence electrons each). . C. . : . . F. to write a Lewis structure for CF 4. . . : . . F: . . : : F : . . F: C. . : . . F: The octet rule is satisfied for carbon and each fluorine.
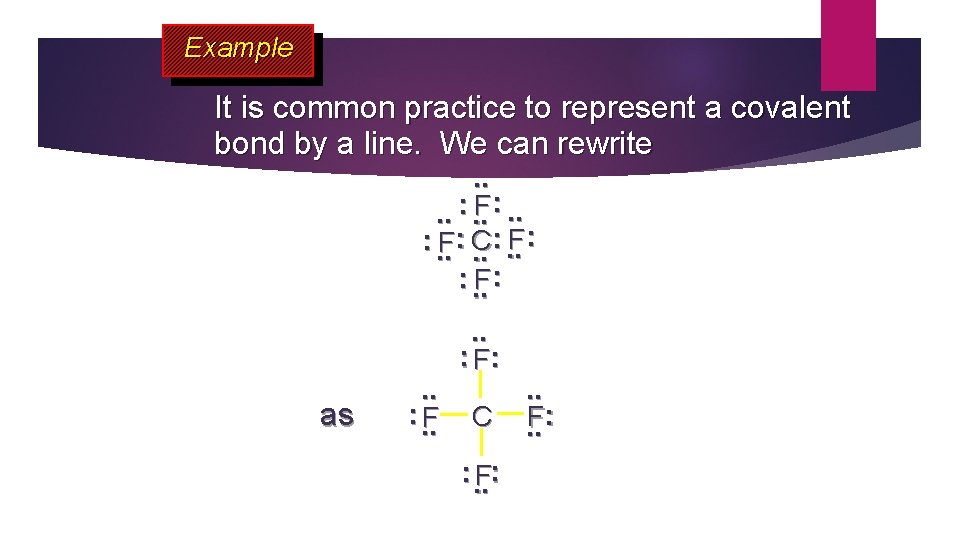
Example It is common practice to represent a covalent bond by a line. We can rewrite. . : . . F: . . : : F : . . F: C. . : . . F: as . . : . . F . . : F: C : . . F:
Multiple Covalent Bonds Sometimes atoms attain noble gas configuration by sharing 2 or 3 pairs of electrons Double covalent bond and Triple covalent bond
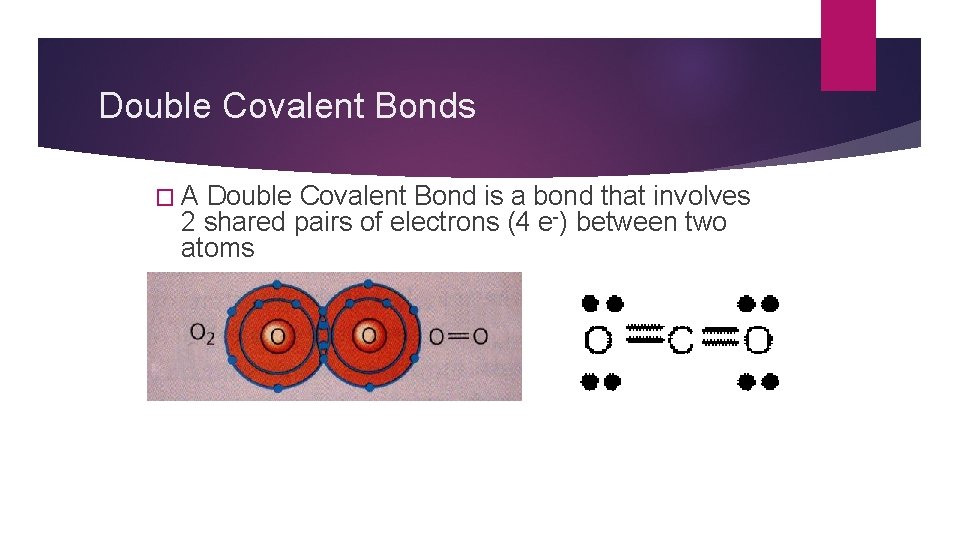
Double Covalent Bonds �A Double Covalent Bond is a bond that involves 2 shared pairs of electrons (4 e-) between two atoms

O 2 Oxygen is also one of the diatomic molecules
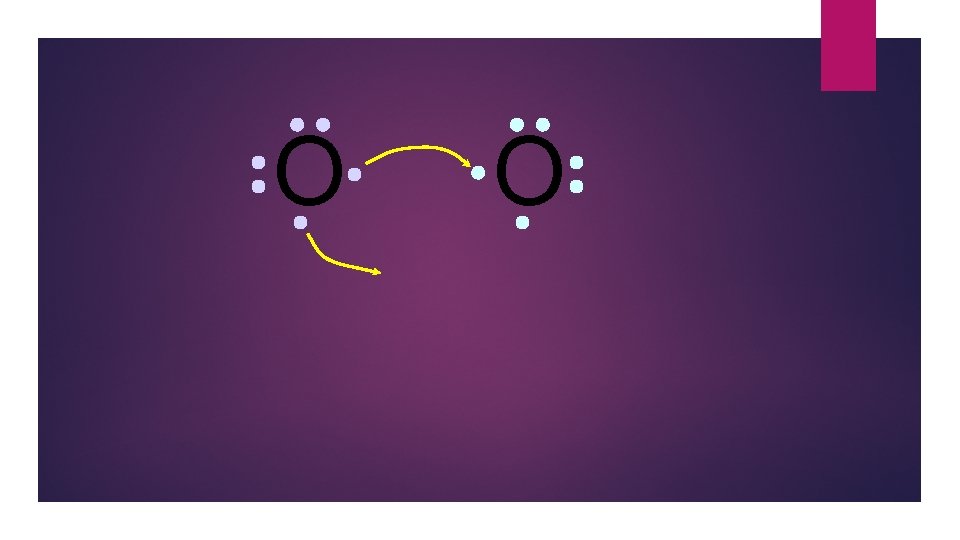
O O How will two oxygen atoms bond?
O O Each atom has two unpaired electrons
O O
O O
O O
O O
O O
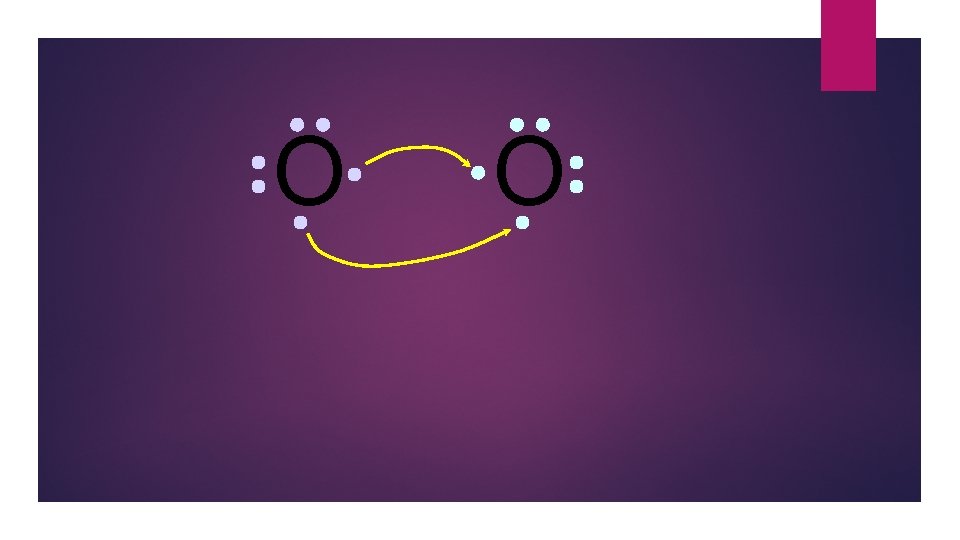
O O
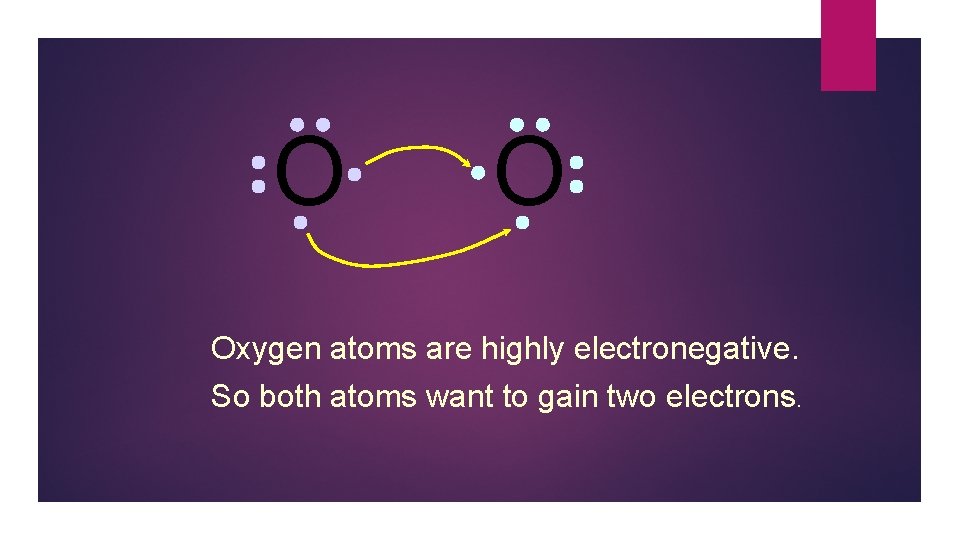
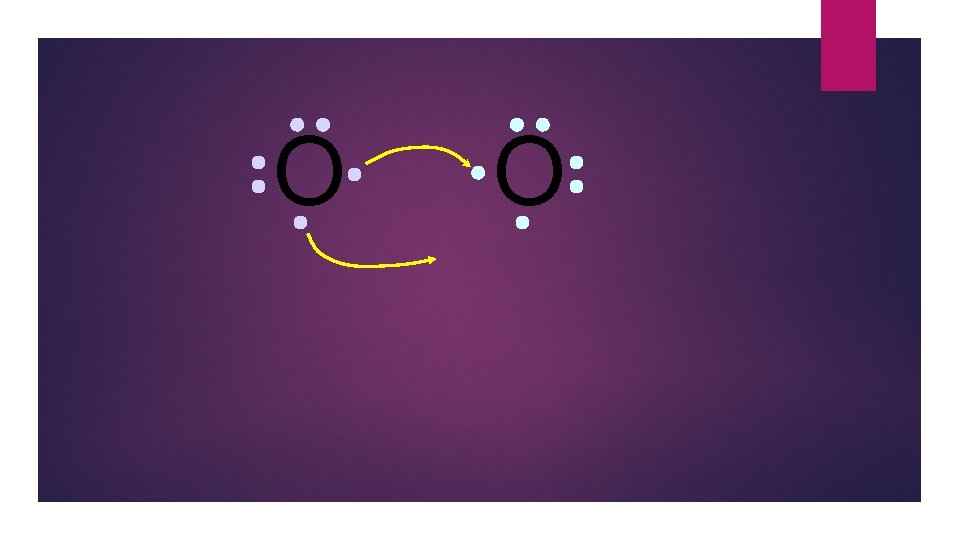
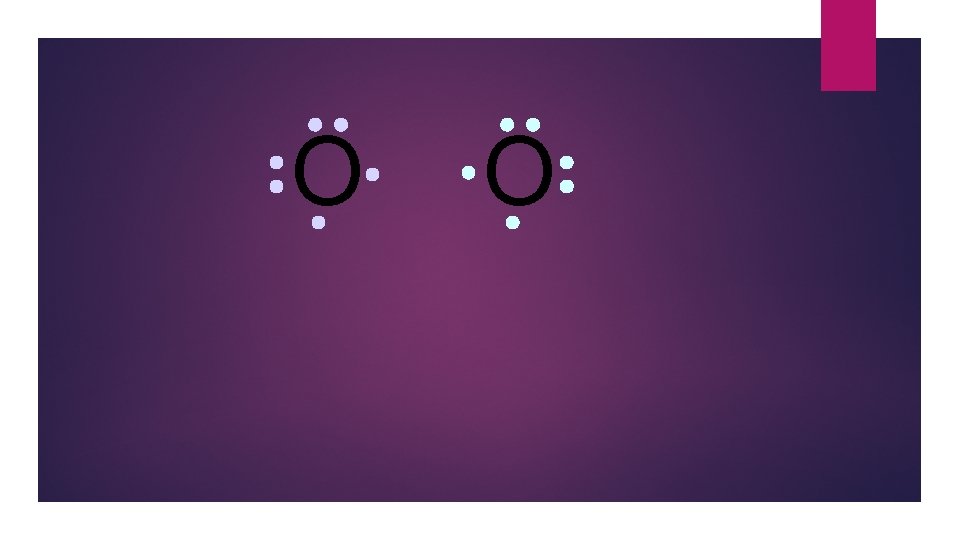
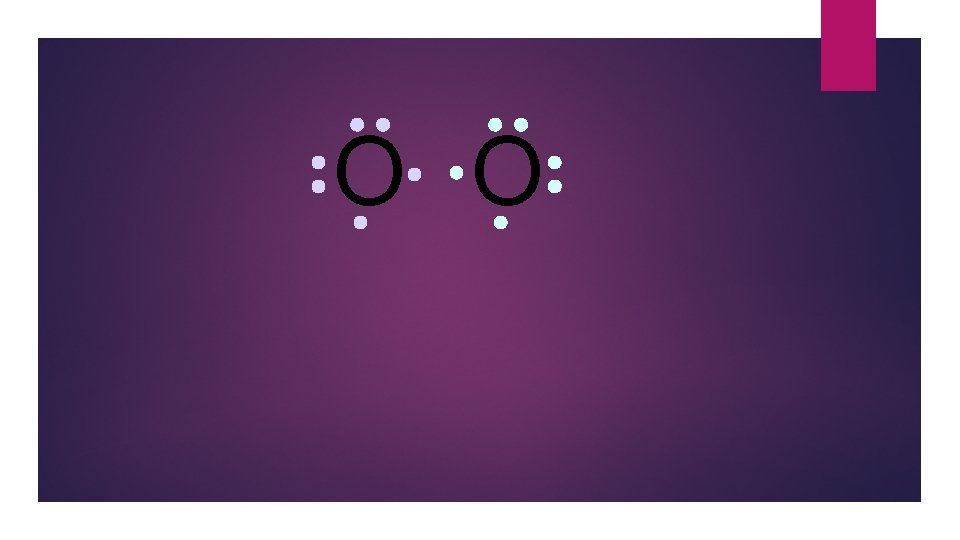
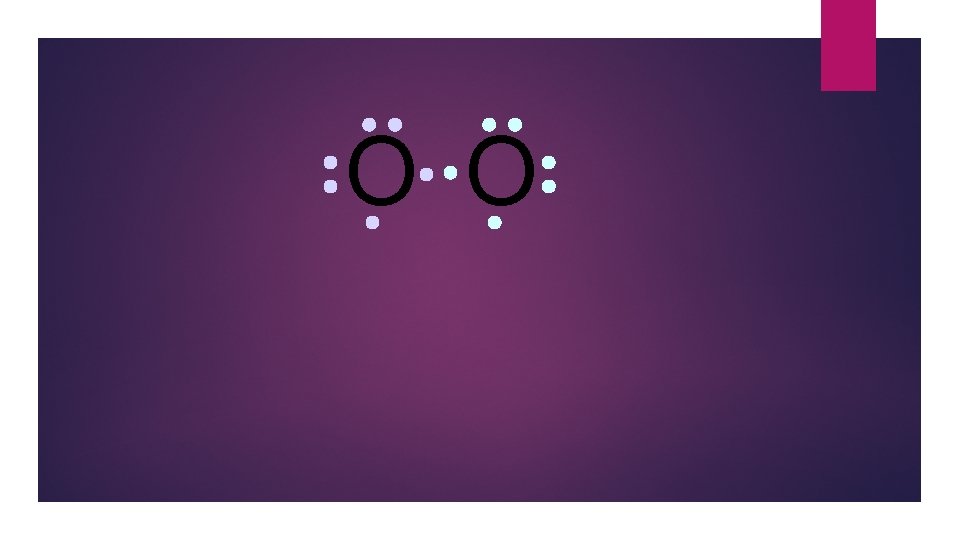
O O Oxygen atoms are highly electronegative. So both atoms want to gain two electrons.
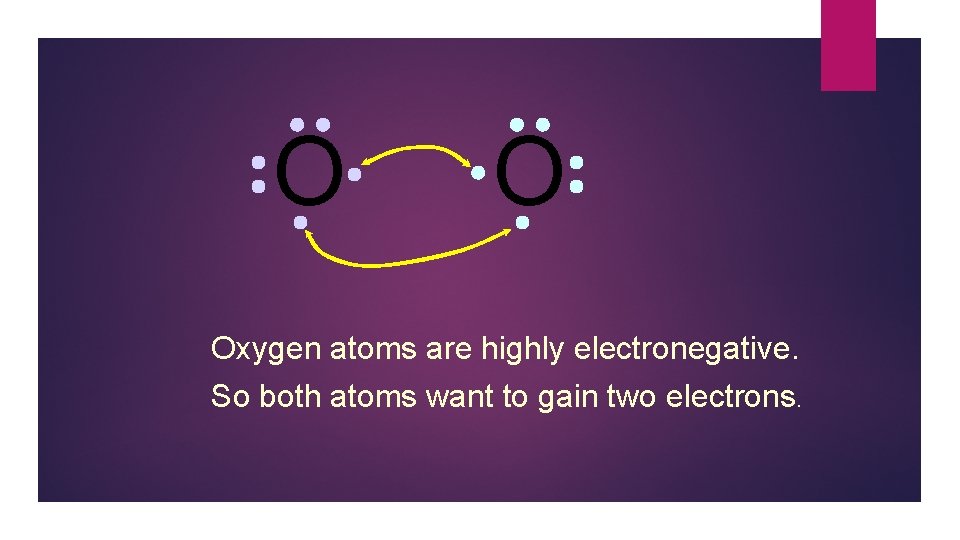
O O Oxygen atoms are highly electronegative. So both atoms want to gain two electrons.
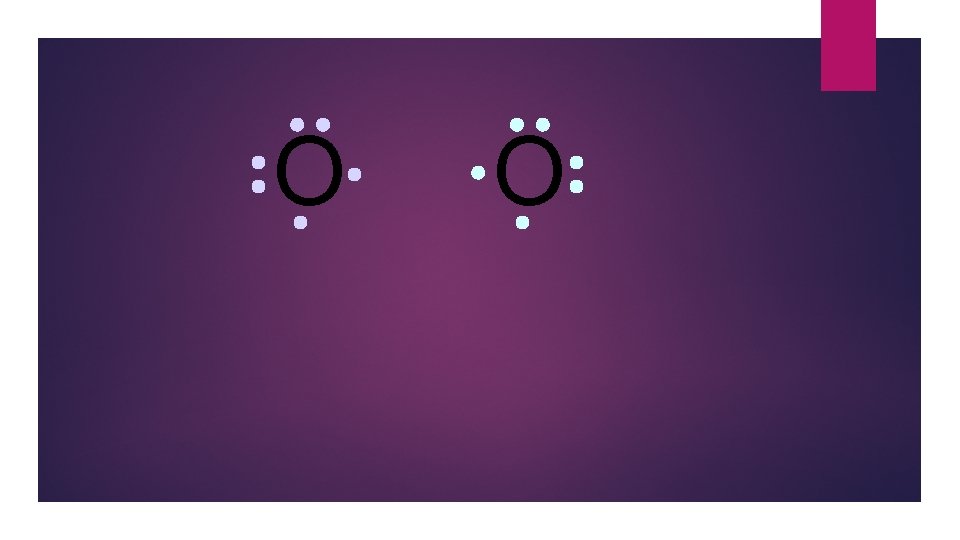
O O
O O
O O
O O
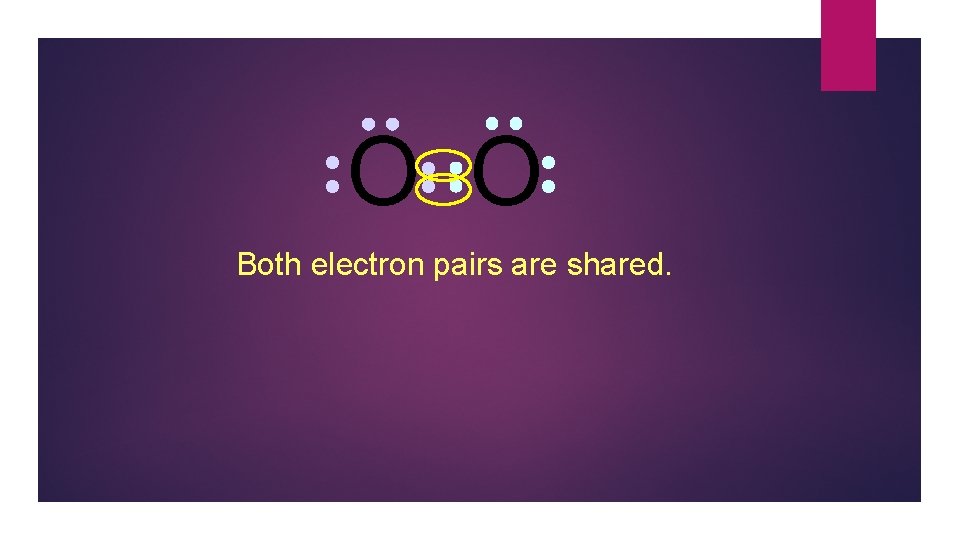
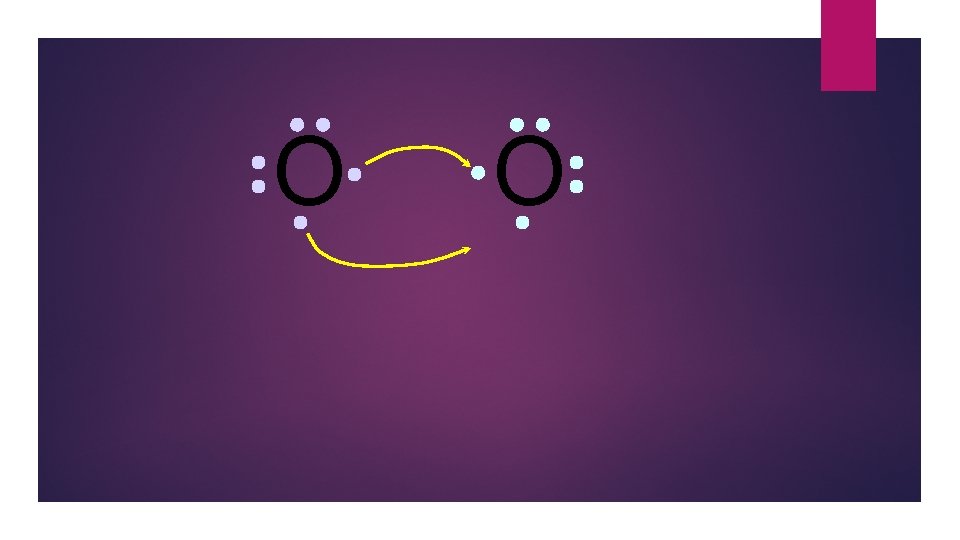
O O Both electron pairs are shared.
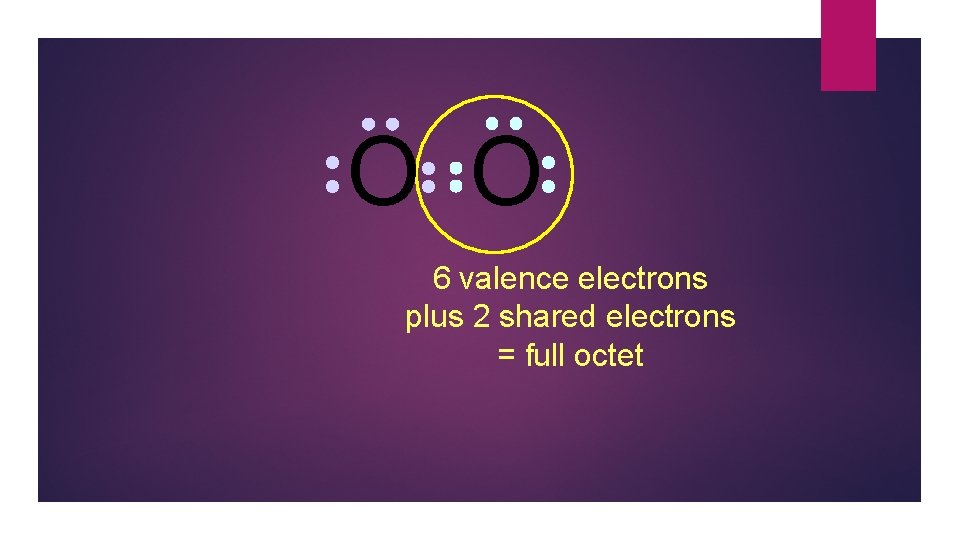
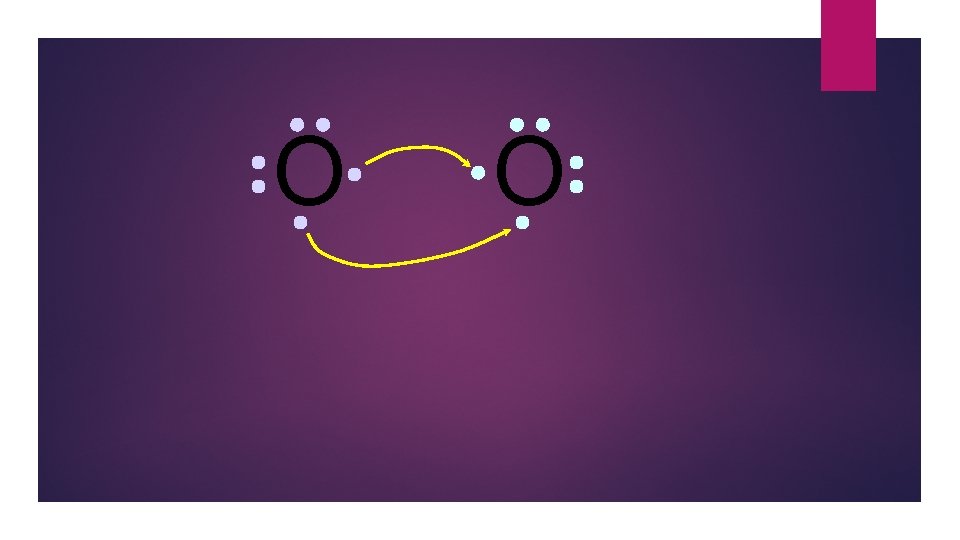
O O 6 valence electrons plus 2 shared electrons = full octet
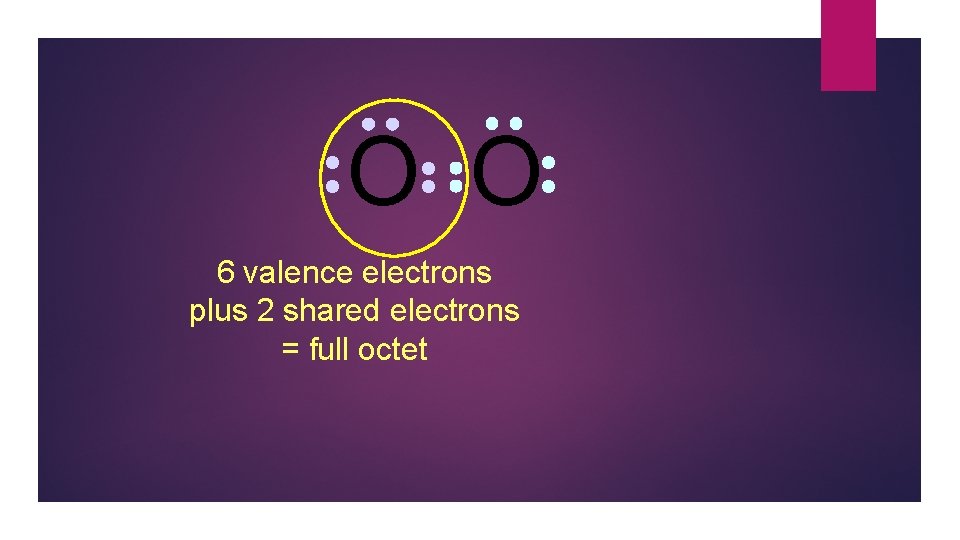
O O 6 valence electrons plus 2 shared electrons = full octet
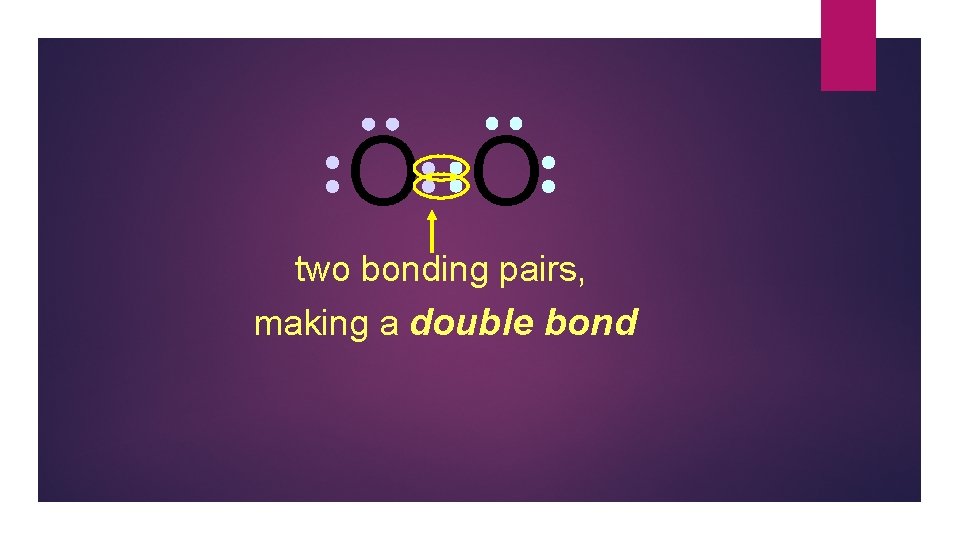
O O two bonding pairs, making a double bond
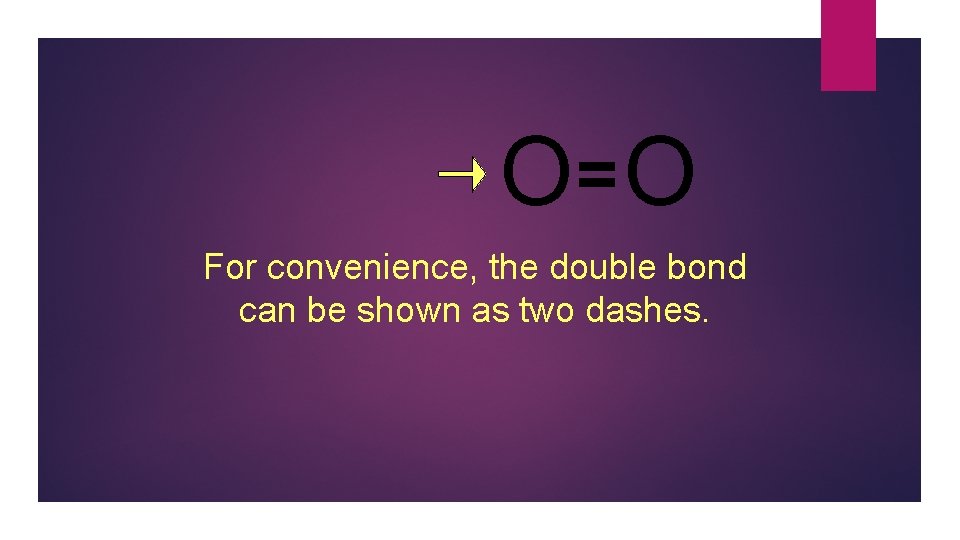
O= O For convenience, the double bond can be shown as two dashes.

O= O This is the oxygen molecule, O 2 this is so cool!!
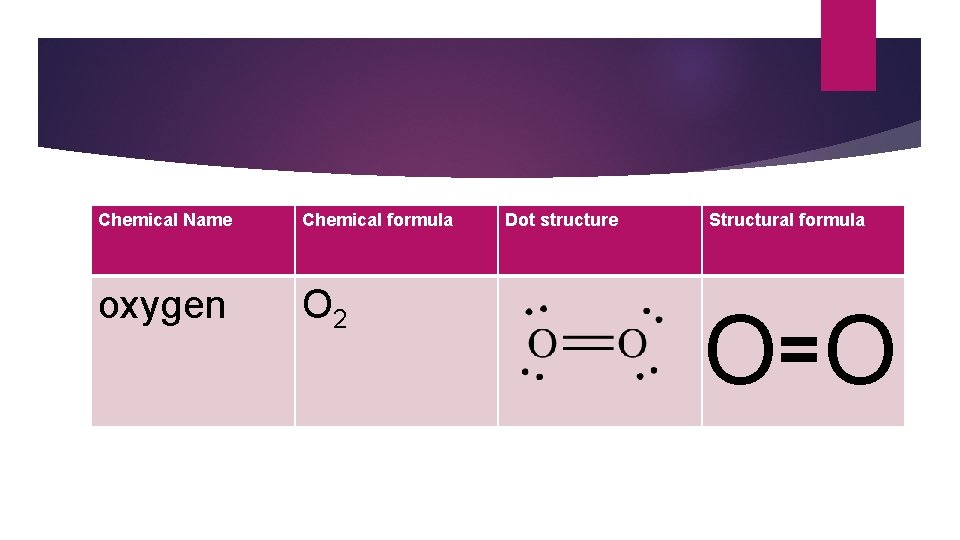
Chemical Name Chemical formula oxygen O 2 Dot structure Structural formula O= O
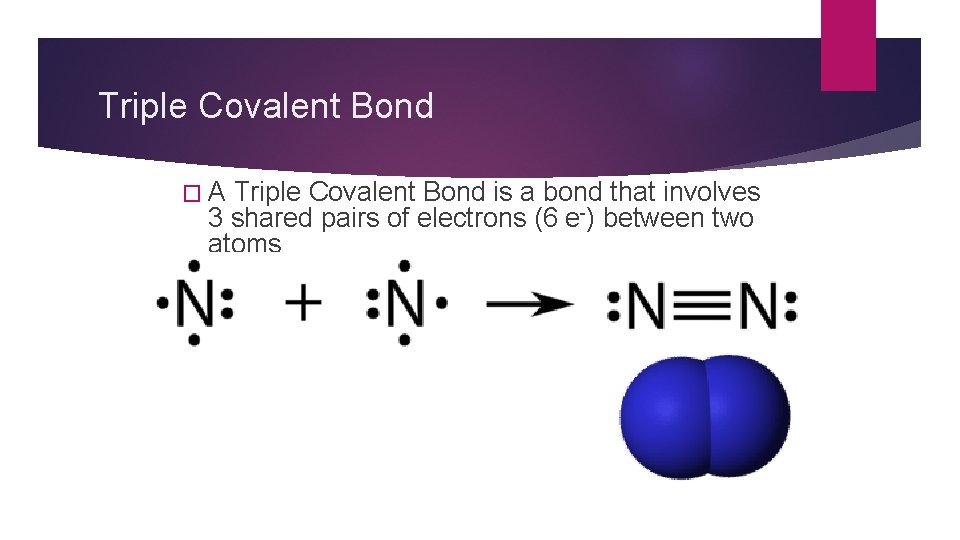
Triple Covalent Bond �A Triple Covalent Bond is a bond that involves 3 shared pairs of electrons (6 e-) between two atoms

Summary �In your notebooks explain why covalent bonds are formed. State the difference between a single, double and triple bond.

General Rules 1. The central atom would usually be the less electronegative atom. 2. Hydrogen and group 17 elements are never central atoms. 3. Make sure all atoms have a full outer shell ( for Hydrogen 2 valence electrons, for all other elements 8 valence electrons)
Create a Table Molecular Dot Formula Structure of Atoms Present Dot Structural Types of Structure Formula covalent of bonds Molecule present
Practice Lewis Dot Structure Group 1: H 2 N 2 O 2 Cl 2 Br 2 I 2 Group 2: F 2 HCl CH 4 H 2 O NH 3 H 2 Se Group 3: HF CO 2 NI 3 CBr 4 HCN H 2 S
- Slides: 70