Aim to develop a clinical practice guideline for














- Slides: 33


Aim: to develop a clinical practice guideline for diagnosis of PCD, which will determine who should be tested evaluate the evidence base for 5 diagnostic methods. develop an algorithm for: a) a definite diagnosis of PCD; b) a probable diagnosis of PCD; c) exclusion of PCD ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 2

What is PCD? ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 3

Primary Ciliary Dyskinesia • Rare heterogeneous condition • Usually inherited in autosomal recessive pattern • Incidence ≈1: 10, 000 • There is no ‘gold standard’ diagnostic test • Diagnosis depends on a number of complicated, expensive tests • Some of these tests are not available in all countries ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 4

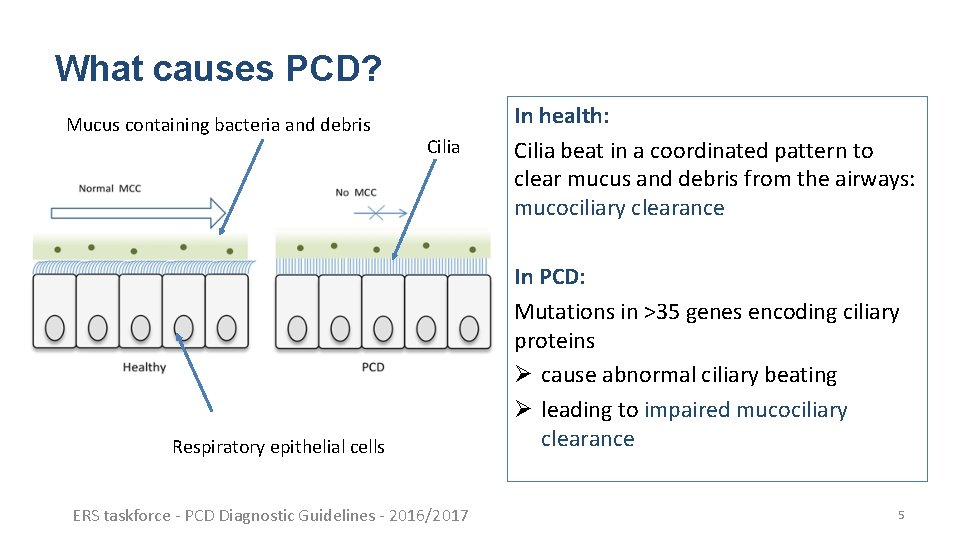
What causes PCD? Mucus containing bacteria and debris Cilia Respiratory epithelial cells ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 In health: Cilia beat in a coordinated pattern to clear mucus and debris from the airways: mucociliary clearance In PCD: Mutations in >35 genes encoding ciliary proteins Ø cause abnormal ciliary beating Ø leading to impaired mucociliary clearance 5

Impaired mucociliary clearance and ciliary dysfunction in patients with PCD can cause • Persistent upper and lower airway symptoms from early infancy • Conductive hearing loss, bronchiectasis and eventually respiratory failure • Situs abnormalities in 50%, more rarely cardiac defects • Fertility problems, particularly in men ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 6

ERS Guideline for the diagnosis of PCD ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 7

Cite this article as: Lucas JS, Barbato A, Collins SA, et al. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur Respir J 2017; 49: 1601090 [https: //doi. org/10. 1183/13993003. 01090 -2016]. ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 8

Strength of evidence was based on the GRADE approach • Grading of • Recommendations • Applicability • Development and • • • Formulate questions Systematic literature review Quality of evidence Data synthesis Recommendations – strong or weak • Evaluation Schunemann et al, Am J Respir Crit Care Med 2006 ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 9

Which patients should be referred for PCD diagnostic testing? ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 10

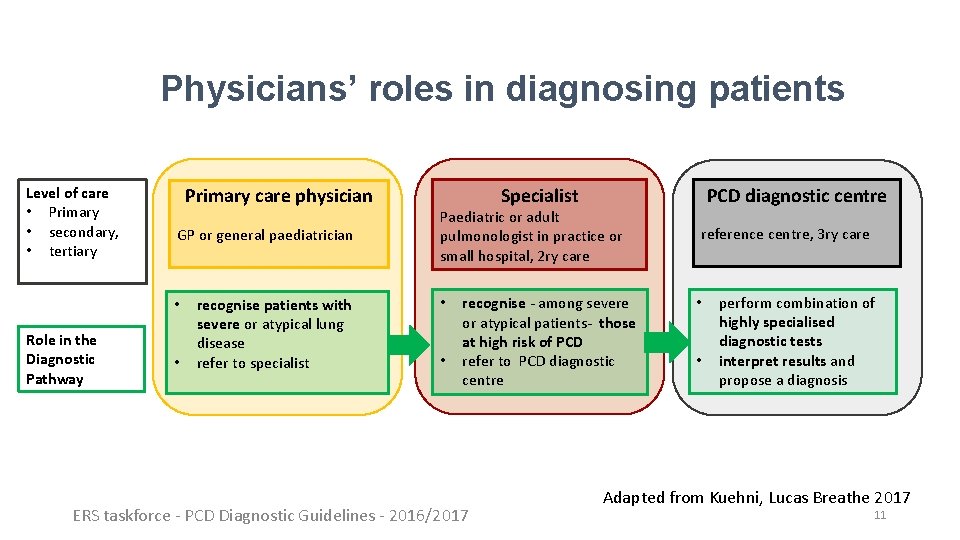
Physicians’ roles in diagnosing patients Level of care • Primary • secondary, • tertiary Primary care physician GP or general paediatrician • Role in the Diagnostic Pathway • recognise patients with severe or atypical lung disease refer to specialist Specialist Paediatric or adult pulmonologist in practice or small hospital, 2 ry care • • recognise - among severe or atypical patients- those at high risk of PCD refer to PCD diagnostic centre ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 PCD diagnostic centre reference centre, 3 ry care • • perform combination of highly specialised diagnostic tests interpret results and propose a diagnosis Adapted from Kuehni, Lucas Breathe 2017 11

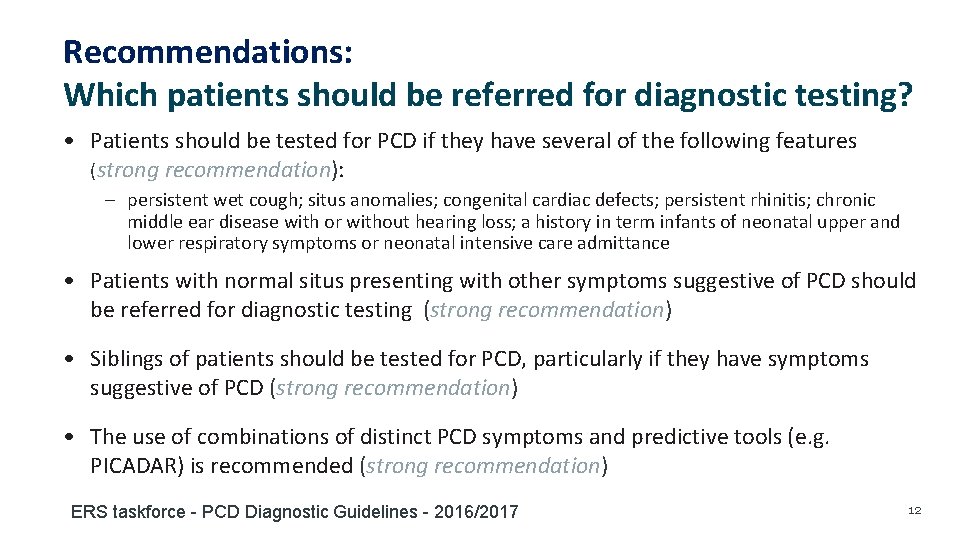
Recommendations: Which patients should be referred for diagnostic testing? • Patients should be tested for PCD if they have several of the following features (strong recommendation): – persistent wet cough; situs anomalies; congenital cardiac defects; persistent rhinitis; chronic middle ear disease with or without hearing loss; a history in term infants of neonatal upper and lower respiratory symptoms or neonatal intensive care admittance • Patients with normal situs presenting with other symptoms suggestive of PCD should be referred for diagnostic testing (strong recommendation) • Siblings of patients should be tested for PCD, particularly if they have symptoms suggestive of PCD (strong recommendation) • The use of combinations of distinct PCD symptoms and predictive tools (e. g. PICADAR) is recommended (strong recommendation) ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 12

Which diagnostic tests should be used? ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 13

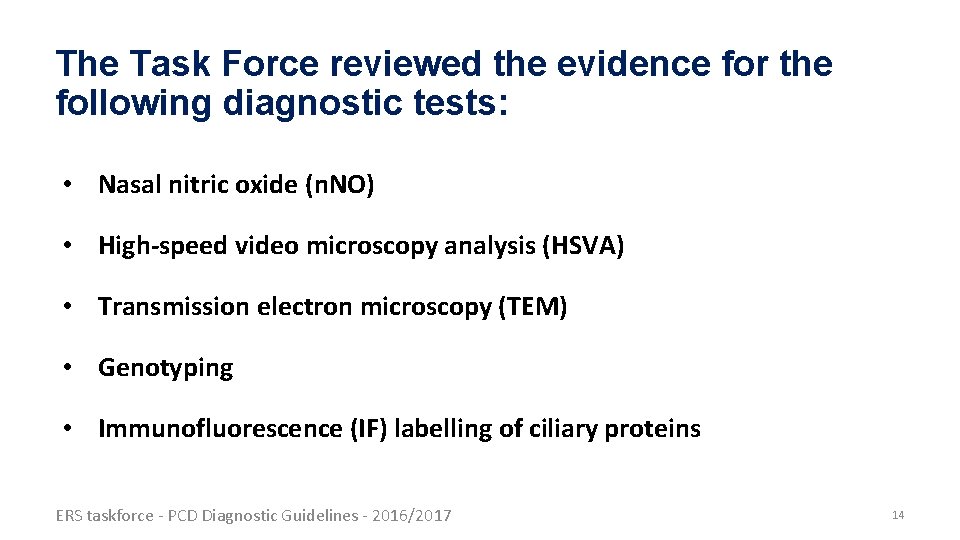
The Task Force reviewed the evidence for the following diagnostic tests: • Nasal nitric oxide (n. NO) • High-speed video microscopy analysis (HSVA) • Transmission electron microscopy (TEM) • Genotyping • Immunofluorescence (IF) labelling of ciliary proteins ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 14

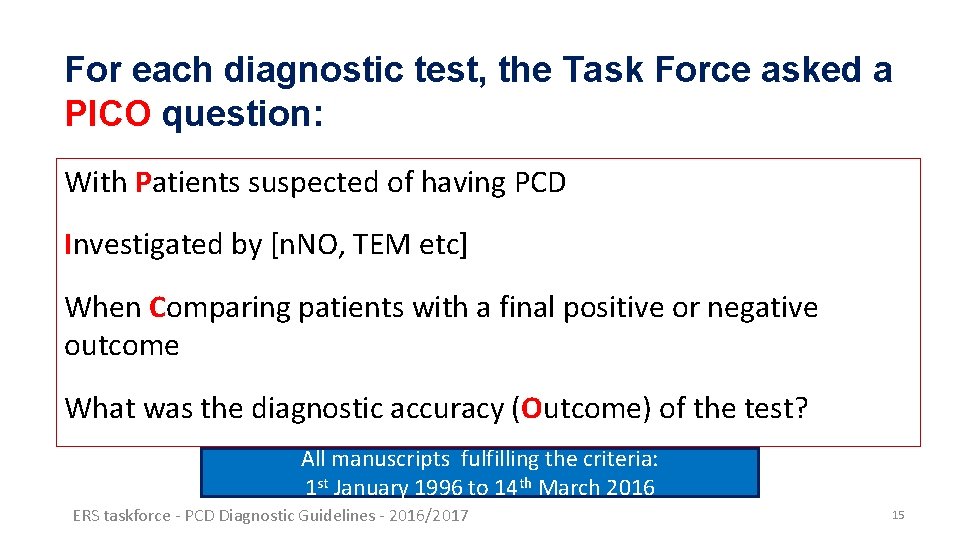
For each diagnostic test, the Task Force asked a PICO question: With Patients suspected of having PCD Investigated by [n. NO, TEM etc] When Comparing patients with a final positive or negative outcome What was the diagnostic accuracy (Outcome) of the test? All manuscripts fulfilling the criteria: 1 st January 1996 to 14 th March 2016 ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 15

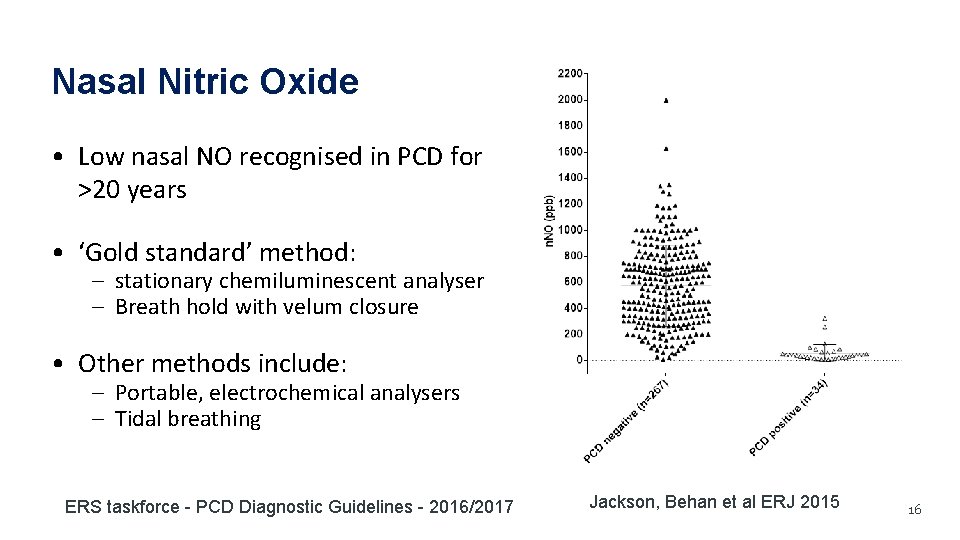
Nasal Nitric Oxide • Low nasal NO recognised in PCD for >20 years • ‘Gold standard’ method: – stationary chemiluminescent analyser – Breath hold with velum closure • Other methods include: – Portable, electrochemical analysers – Tidal breathing ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 Jackson, Behan et al ERJ 2015 16

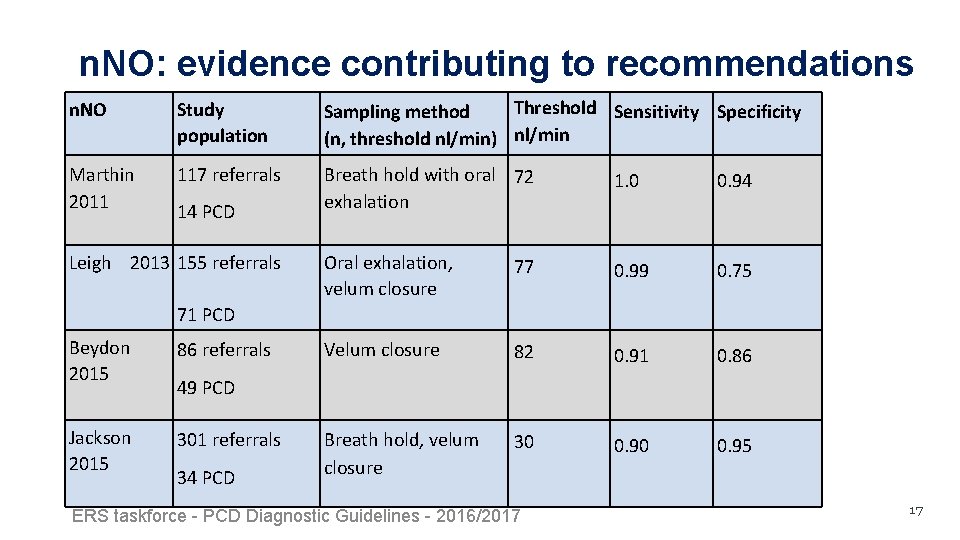
n. NO: evidence contributing to recommendations n. NO Study population Threshold Sensitivity Specificity Sampling method (n, threshold nl/min) nl/min Marthin 2011 117 referrals Breath hold with oral 72 exhalation 1. 0 0. 94 Oral exhalation, velum closure 77 0. 99 0. 75 Velum closure 82 0. 91 0. 86 Breath hold, velum closure 30 0. 95 14 PCD Leigh 2013 155 referrals 71 PCD Beydon 2015 86 referrals Jackson 2015 301 referrals 49 PCD 34 PCD ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 17

Recommendations: Nasal nitric oxide n. NO has good sensitivity and specificity It is relatively easy to perform and non-invasive The panel considered that it should be used as part of the diagnostic work-up of patients suspected of having PCD, with testing performed by individuals who are trained to take measurements and interpret results • >6 years: n. NO should be used as part of the diagnostic work-up, preferably using a chemiluminescence analyser with velum closure (strong recommendation) • <6 years: suggested n. NO measurement using tidal breathing is part of the diagnostic work-up (weak recommendation) • n. NO is not sufficiently accurate to rule PCD in or out in isolation. Therefore patients with a strong clinical history should undergo further testing, even if n. NO is normal (weak recommendation) ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 18

High Speed Video Analysis (HSVA) • HSVA is used to assess – ciliary beat frequency – ciliary beat pattern • Samples are obtained from respiratory epithelium of the nose or bronchus • HSVA methods are not standardized • Re-analysis following culture is a technique used to mitigate secondary ciliary defects ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 19

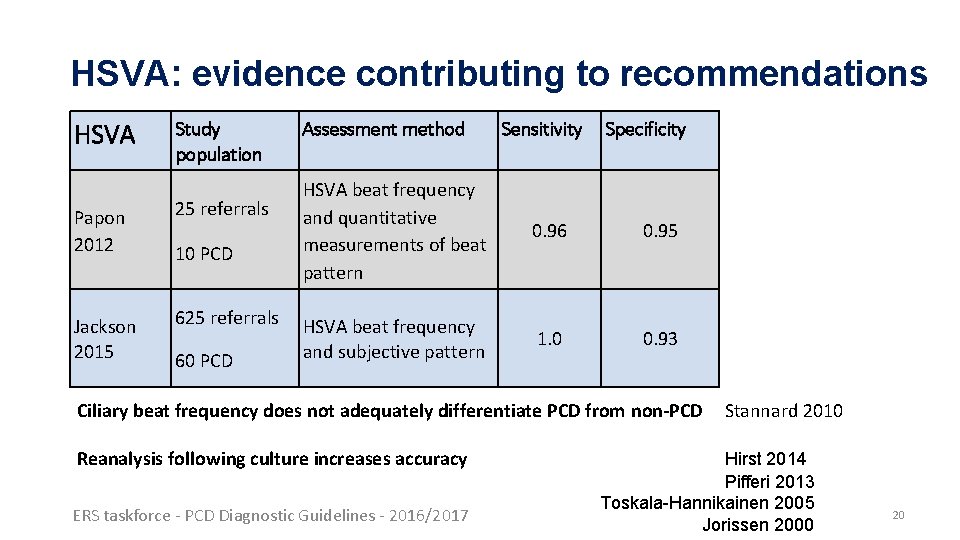
HSVA: evidence contributing to recommendations HSVA Study population Papon 2012 25 referrals Jackson 2015 625 referrals 10 PCD 60 PCD Assessment method Sensitivity Specificity HSVA beat frequency and quantitative measurements of beat pattern 0. 96 0. 95 HSVA beat frequency and subjective pattern 1. 0 0. 93 Ciliary beat frequency does not adequately differentiate PCD from non-PCD Reanalysis following culture increases accuracy ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 Stannard 2010 Hirst 2014 Pifferi 2013 Toskala-Hannikainen 2005 Jorissen 2000 20

Recommendations: High speed video-microscopy analysis The expert panel considered that HSVA should be performed by experienced staff as part of the diagnostic work-up of patients suspected of having PCD. • • • HSVM including ciliary beat frequency and pattern should be used as part of the diagnostic work-up (weak recommendation) Ciliary beat frequency should not be used without assessment of ciliary beat pattern (strong recommendation) To improve diagnostic accuracy CBF/P assessment should be repeated after ALI culture (strong recommendation) ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 21

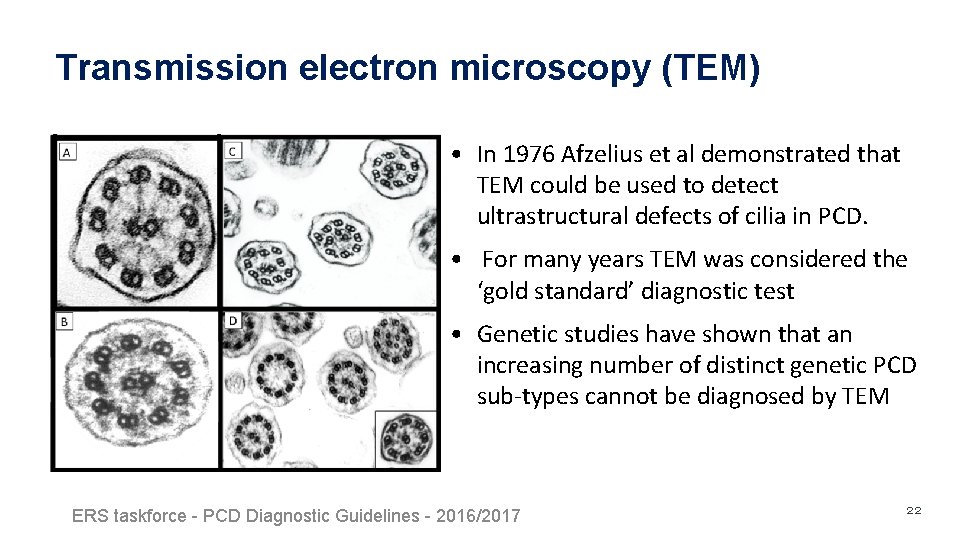
Transmission electron microscopy (TEM) • In 1976 Afzelius et al demonstrated that TEM could be used to detect ultrastructural defects of cilia in PCD. • For many years TEM was considered the ‘gold standard’ diagnostic test • Genetic studies have shown that an increasing number of distinct genetic PCD sub-types cannot be diagnosed by TEM ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 22

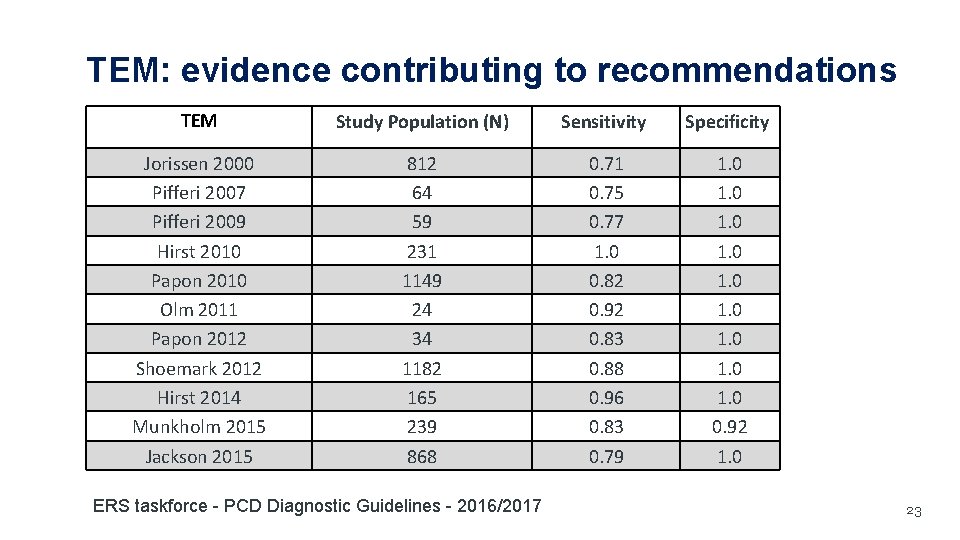
TEM: evidence contributing to recommendations TEM Study Population (N) Sensitivity Specificity Jorissen 2000 Pifferi 2007 Pifferi 2009 Hirst 2010 Papon 2010 Olm 2011 Papon 2012 Shoemark 2012 Hirst 2014 Munkholm 2015 Jackson 2015 812 64 59 231 1149 24 34 1182 165 239 868 0. 71 0. 75 0. 77 1. 0 0. 82 0. 92 0. 83 0. 88 0. 96 0. 83 0. 79 1. 0 1. 0 0. 92 1. 0 ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 23

Recommendations: Transmission electron microscopy The expert panel considered that TEM should be performed by experienced staff as part of the diagnostic work-up of patients suspected of having PCD. • • • Ciliary ultrastructure analysis by TEM should be used as part of the diagnostic work-up (strong recommendation) Further diagnostic tests should be performed in patients with normal TEM if the history is strong (strong recommendation) In patients with hallmark ciliary ultrastructure defects: further tests are not required (strong recommendation) ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 24

Genetic testing for PCD • PCD is genetically heterogeneous, with >30 causative genes • It is usually inherited in an autosomal recessive pattern • No studies had properly evaluated the value of genotyping in a diagnostic setting • Several studies suggest that genetic testing identifies ≈65% of cases • Genetic testing can confirm the diagnosis ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 25

Genetic testing for PCD The role of genetic testing as a diagnostic tool for PCD could not be evaluated by the Task Force in an evidence-based way, as there were no studies designed to assess this question. Whilst further evidence in a diagnostic setting is required, the expert panel agreed: • Genetic testing can be used to confirm diagnosis in people diagnosed by other means (e. g. HSVA) or in patients with high clinical suspicion for PCD and no availability of other tests, such as HSVA, TEM or IF. • Genetic testing can help to establish diagnosis in patients highly suspected of PCD and in whom HSVA, TEM or IF failed to confirm the diagnosis. • A negative genetic test does not exclude PCD. • Genetic diagnosis has to be consistent with the clinical and TEM/IF/HSVA phenotype, or diagnosis reconsidered if the picture is inconsistent. • Allelic segregation analysis within the family is important to confirm the genotype in the probands. ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 26

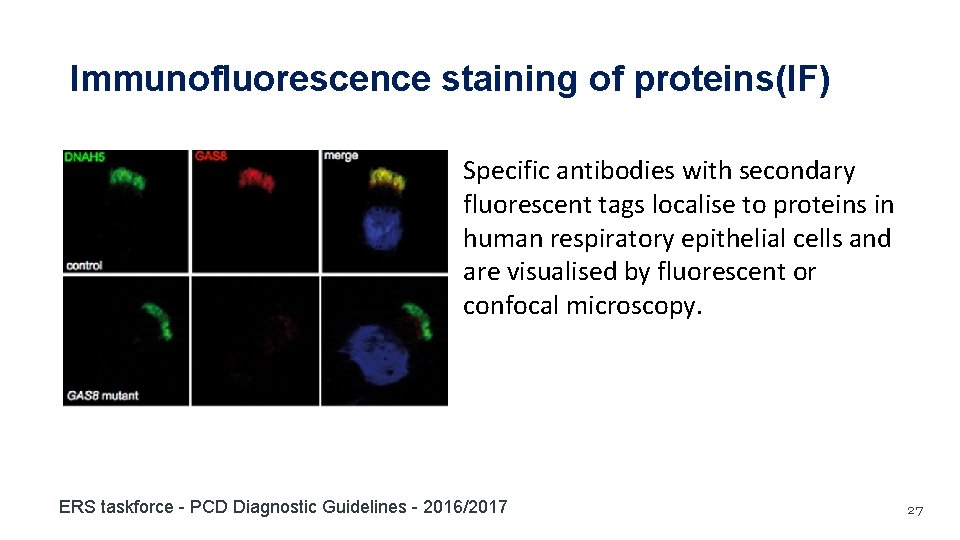
Immunofluorescence staining of proteins(IF) Specific antibodies with secondary fluorescent tags localise to proteins in human respiratory epithelial cells and are visualised by fluorescent or confocal microscopy. ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 27

IF testing for PCD The role of genetic testing as a diagnostic tool for PCD could not be evaluated by the Task Force in an evidence-based way, as there were no studies designed to assess this question. Whilst further evidence in a diagnostic setting is required, the expert panel agreed: – IF is able to confirm pathogenesis of mutations – IF can detect some PCD cases with normal TEM – IF can help establish the diagnosis in some patients ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 28

Has the patient got PCD? ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 29

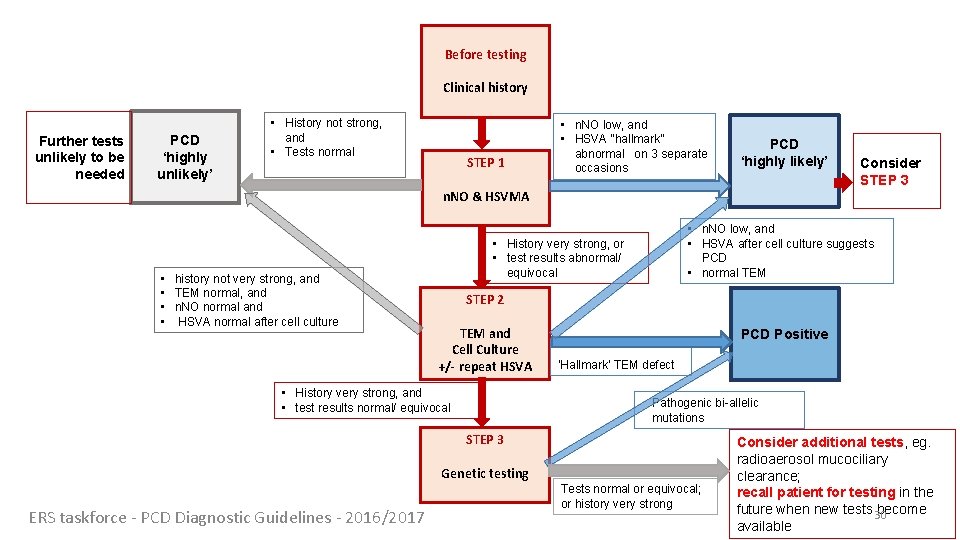
Before testing Clinical history Further tests unlikely to be needed PCD ‘highly unlikely’ • History not strong, and • Tests normal STEP 1 • n. NO low, and • HSVA “hallmark” abnormal on 3 separate occasions PCD ‘highly likely’ n. NO & HSVMA • history not very strong, and • TEM normal, and • n. NO normal and • HSVA normal after cell culture • n. NO low, and • HSVA after cell culture suggests PCD • normal TEM • History very strong, or • test results abnormal/ equivocal STEP 2 TEM and Cell Culture +/- repeat HSVA • History very strong, and • test results normal/ equivocal PCD Positive ‘Hallmark’ TEM defect Pathogenic bi-allelic mutations STEP 3 Genetic testing ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 Consider STEP 3 Tests normal or equivocal; or history very strong Consider additional tests, eg. radioaerosol mucociliary clearance; recall patient for testing in the future when new tests 30 become available

Summary • There is no ‘gold standard’ and diagnosis depends on a combination of tests • Analyses and interpretation should be conducted by PCD experts • Even in expert centres the diagnostic outcome of some patients will remain uncertain • The Task Force recommended a research road map to include standardisation of testing and reporting • The Task Force recommended that the Guideline should be reviewed in 3 -5 years ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 31

Slides made by: Jane Lucas 1 Angelo Barbato 2 and Claudia Kuehni 3 1. University of Southampton, UK jlucas 1@soton. ac. uk 2. University of Padova, Italy angelo. barbato 45@gmail. com 3. Institute of Social and Preventive Medicine, University of Bern, Switzerland claudia. kuehni@ispm. unibe. ch With the help of: • Alex Gileles-Hillel, Hadassah Hebrew University, Jerusalem, Israel. • • • Amparo Escribano, University Clinic Hospital, University of Valencia. Spain. Azevedo Ines, Department of Paediatrics, University of Porto, Portugal. Constant Carolina, Paediatric Pulmonology Unit, Hospital de Santa Maria, Lisbon. Castillo Corullón Silvia, University Clinic Hospital, University of Valencia. Spain Karadag Bulent, Pediatric Pulmonology, Marmara University, Istanbul, Turkey. Romero Rubio Maria Teresa, Working Group on PCD, University Hospital Valencia, Spain. Nisreen Rumman, Makassed Hospital, East Jerusalem, Palestine Rovira Amigo, Sandra, Department of Paediatrics, Hospital Vall d’Hebron, Barcelona, Spain Vizmanos-Lamotte Gerardo, CMQ, Andorra ERS taskforce - PCD Diagnostic Guidelines - 2016/2017

ERS PCD Task Force JS Lucas, University of Southampton and University Hospital Southampton, UK; A Barbato, University of Padova, Italy; SA Collins, University of Southampton, UK; M Goutaki, University of Bern, Switzerland; L Behan, University of Southampton, UK; D Caudri, The University of Western Australia, Subiaco, Australia; S Dell, University of Toronto, Ontario, Canada; E Eber, Medical University of Graz, Austria; E Escudier, Sorbonne Universités (UPMC Univ Paris 06), Paris, France; R Hirst, Leicester Royal Infirmary, Leicester, UK; C Hogg, Imperial College and Royal Brompton Hospital, London UK; M Jorissen, KULeuven, Belgium; P Latzin, University of Bern, Switzerland; M Legendre, Sorbonne Universités (UPMC Univ Paris 06), Paris, France; MW Leigh, University of North Carolina at Chapel Hill, USA; F Midulla, Sapienza University of Rome, Italy; K Nielsen, Copenhagen University Hospital, Rigshospitalet, Denmark; H Omran, University Hospital Muenster, Muenster Germany; J-F Papon, Université Paris-Sud, France; P Pohunek, Charles University and Motol University Hospital, Prague 5, Czech Republic; B Redfern, Patient Representative, PCD Family Support Group, UK; D Rigau, Iberoamerican Cochrane Center, Barcelona, Spain; B Rindlisbacher, German speaking European Patient Association based in Germany “Kartagener Syndrom und Primäre Ciliäre Dyskinesie e. V. ” ; F Santamaria, Federico II University, Naples, Italy; A Shoemark, Imperial College and Royal Brompton Hospital, London UK; D Snijders, University of Padova, Italy; T Tonia, Institute of Social and Preventive Medicine, University of Bern, Switzerland; A Titieni, Department of Pediatrics, University Hospital Muenster, Muenster Germany; WT Walker, University of Southampton and University Hospital Southampton, UK; C Werner, University Hospital Muenster, Muenster Germany; A Bush, Imperial College and Royal Brompton Hospital, London UK; CE Kuehni, University of Bern, Switzerland ERS taskforce - PCD Diagnostic Guidelines - 2016/2017 33