Aim How to write the chemical formula of









- Slides: 22

Aim: How to write the chemical formula of an ionic compound given the name

Naming Ionic Compounds Ionic compounds: usually form between a metal ion and nonmetal ion or a metal ion and a polyatomic ion (from Table E). They consist of positive and negative ions held together by electrostatic forces. Naming ionic compounds requires knowledge of how to name the positive and negative ions that make up the ionic compound “positive ion name + negative ion name”

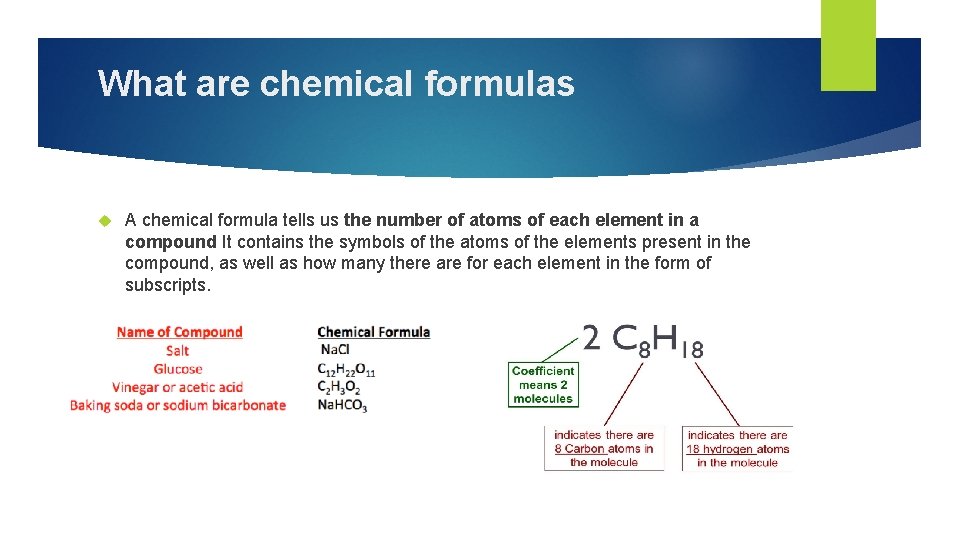
What are chemical formulas A chemical formula tells us the number of atoms of each element in a compound It contains the symbols of the atoms of the elements present in the compound, as well as how many there are for each element in the form of subscripts.

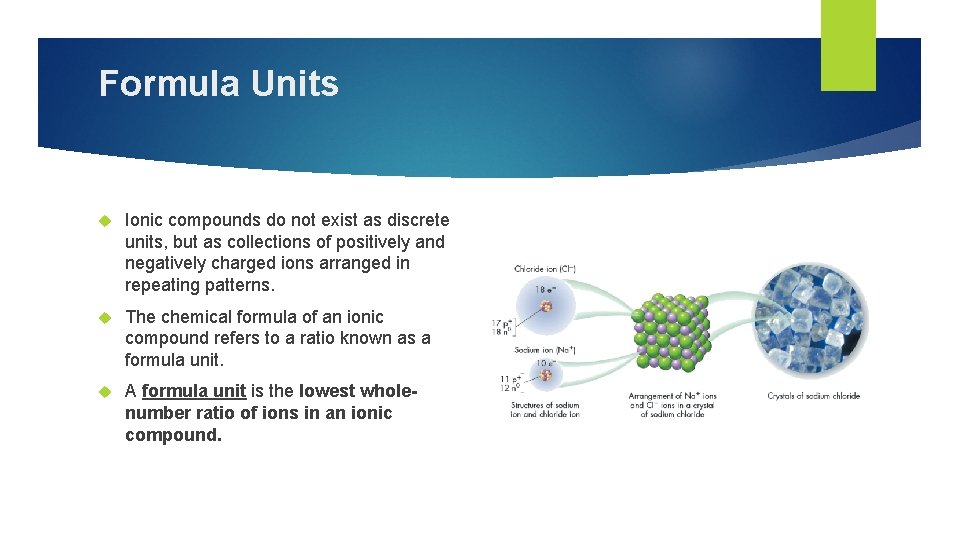
Formula Units Ionic compounds do not exist as discrete units, but as collections of positively and negatively charged ions arranged in repeating patterns. The chemical formula of an ionic compound refers to a ratio known as a formula unit. A formula unit is the lowest wholenumber ratio of ions in an ionic compound.

Formula Unit for Sodium Chloride For sodium chloride, the lowest whole-number ratio of the ions is 1: 1 (one Na+ ion to each Cl– ion). The formula unit for sodium chloride is Na. Cl. Although ionic charges are used to derive the correct formula, they are not shown when you write the formula unit of the compound.

Binary Compounds are composed of two elements bonded together One METAL Ion and One NONMETAL Ion for Ionic Compound Metal atoms lose electrons to form positively charged ions. Some metals form one ion, while others (usually transition metals) can form multiple ions. Nonmetal atoms gain electrons to form negatively charged ions. Nonmetals form only one negative ion. General naming format for binary ionic compounds ion name + nonmetal ion name” “metal

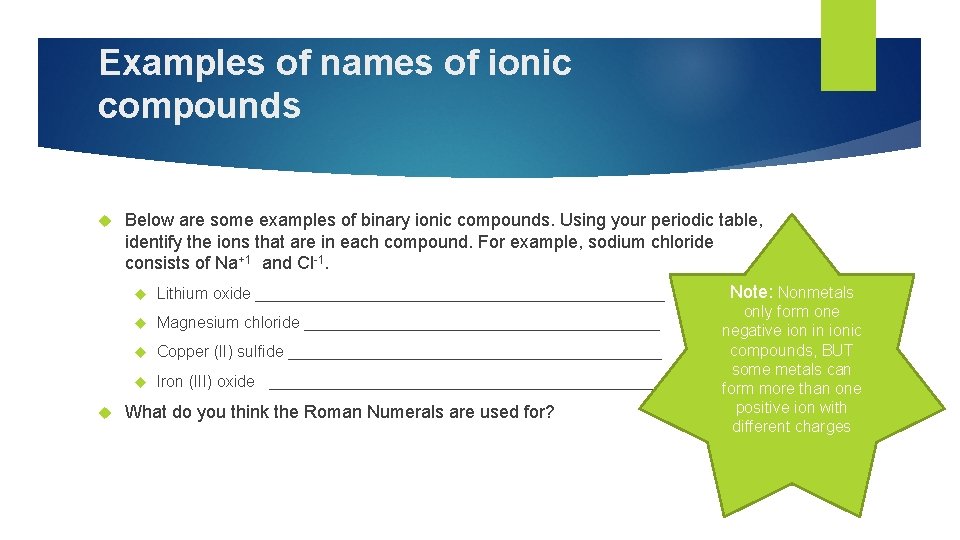
Examples of names of ionic compounds Below are some examples of binary ionic compounds. Using your periodic table, identify the ions that are in each compound. For example, sodium chloride consists of Na+1 and Cl-1. Lithium oxide _______________________ Magnesium chloride ____________________ Copper (II) sulfide _____________________ Iron (III) oxide _______________________ What do you think the Roman Numerals are used for? Note: Nonmetals only form one negative ion in ionic compounds, BUT some metals can form more than one positive ion with different charges

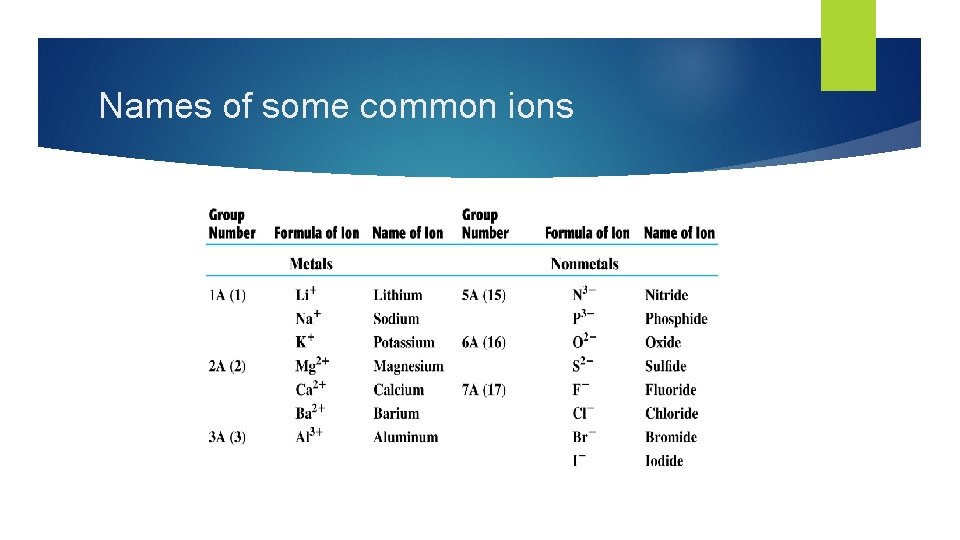
Naming Metal Ions One ion: Some metals form only one ion. Metals of groups 1, 2, 3, 13 & Zn form only one ion each. These ions are named as follows: element name + ion e. g. Na+ = sodium ion Sr 2+ = strontium ion Zn 2+ = zinc ion Multiple ions: Transition metals often form more than one ion. Since these atoms form more than one ion, the charge on the ion must be specified in the name to tell the ions apart. The Stock system is used to name transition metal cations as follows: element name (charge as Roman numeral) + ion e. g. Fe 2+ = iron(II) ion Pb 4+ = lead(IV) ion Cu+ = copper(I) ion

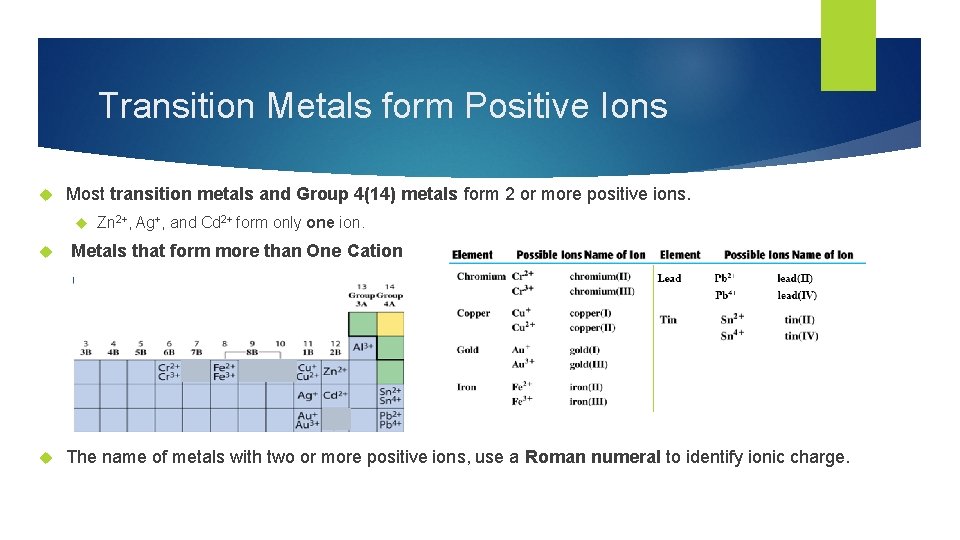
Transition Metals form Positive Ions Most transition metals and Group 4(14) metals form 2 or more positive ions. Zn 2+, Ag+, and Cd 2+ form only one ion. Metals that form more than One Cation The name of metals with two or more positive ions, use a Roman numeral to identify ionic charge.

Name the following metal Ions For some of the metal ions you will need to use the roman numerals in parenthesis. How can you use your periodic table in order to determine if roman numerals are needed? Name the metal ions Ba 2+ _____________ Al 3+ ______________ K+ _______________ Pb 2+ ______________ Cr 3+ ______________

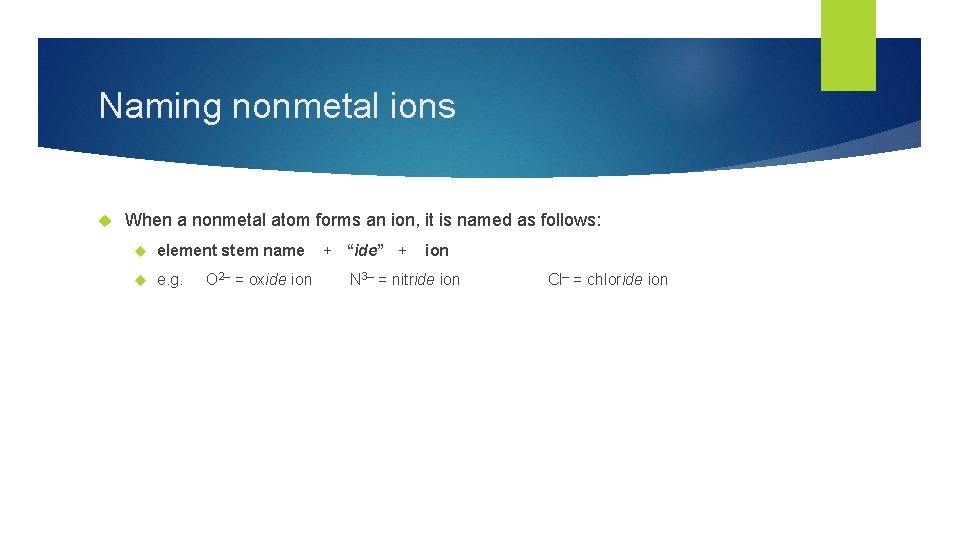
Naming nonmetal ions When a nonmetal atom forms an ion, it is named as follows: element stem name e. g. O 2– = oxide ion + “ide” + ion N 3– = nitride ion Cl– = chloride ion

Names of some common ions

Writing Formulas for Ionic Compounds –Method 1 1. Identify the positive and negative ion. 2. Balances the charges. Remember, ionic compounds are neutral so the sum of the charges of the ions in the ionic compound b=must equal to zero. 3. Write the formula, positive ion first, using subscripts from the balanced equations.

14 Writing Formulas Write a formula for potassium sulfide. 1. Identify the cation and anion. potassium = K+ sulfide = S 2− 2. Balance the charges. K+ S 2− K+ 2(1+) + 1(2 -) = 0 3. 2 K+ and 1 S 2− = K 2 S

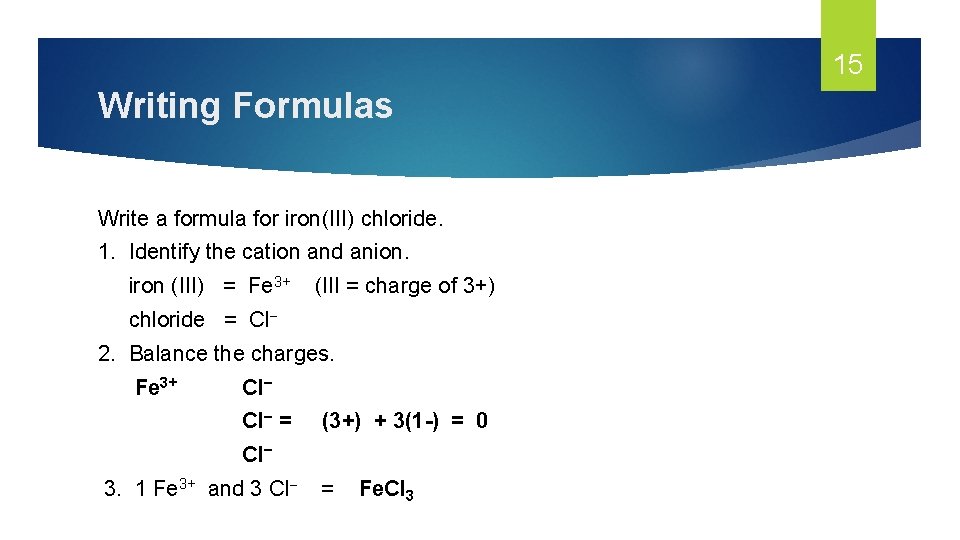
15 Writing Formulas Write a formula for iron(III) chloride. 1. Identify the cation and anion. iron (III) = Fe 3+ (III = charge of 3+) chloride = Cl− 2. Balance the charges. Fe 3+ Cl− = (3+) + 3(1 -) = 0 Cl− 3. 1 Fe 3+ and 3 Cl− = Fe. Cl 3

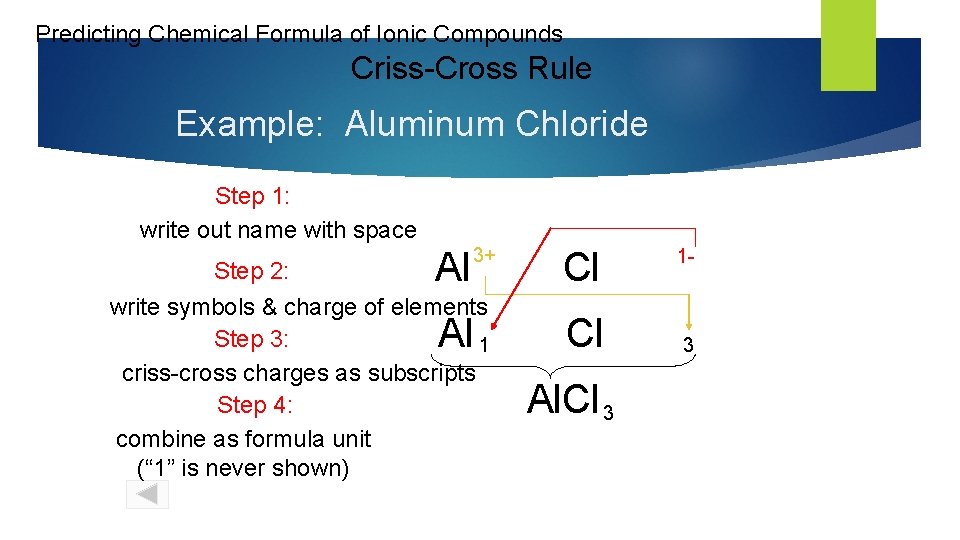
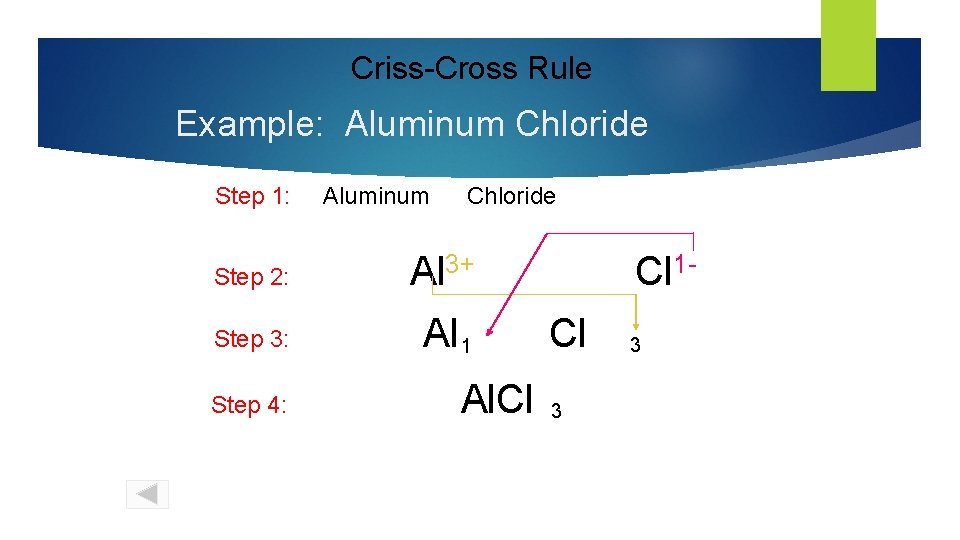
Predicting Chemical Formula of Ionic Compounds Criss-Cross Rule Example: Aluminum Chloride Step 1: write out name with space Al 3+ Step 2: write symbols & charge of elements Step 3: 1 criss-cross charges as subscripts Step 4: combine as formula unit (“ 1” is never shown) Al Cl Cl Al. Cl 3 1 - 3

Criss-Cross Rule Example: Aluminum Chloride Step 1: Aluminum Chloride Step 2: Al 3+ Step 3: Al 1 Step 4: Al. Cl Cl 1 Cl 3 3

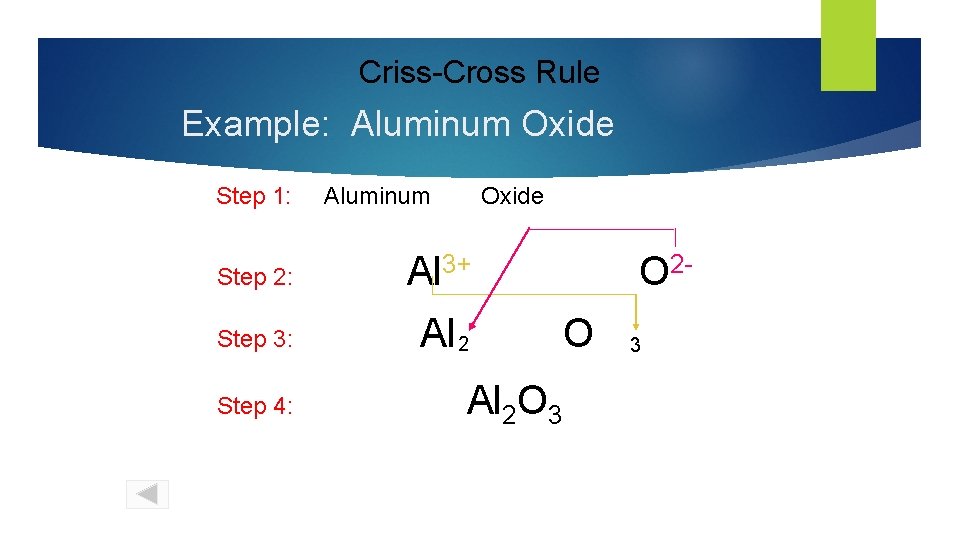
Criss-Cross Rule Example: Aluminum Oxide Step 1: Aluminum Oxide Step 2: Al 3+ Step 3: Al 2 Step 4: Al 2 O 3 O 2 O 3

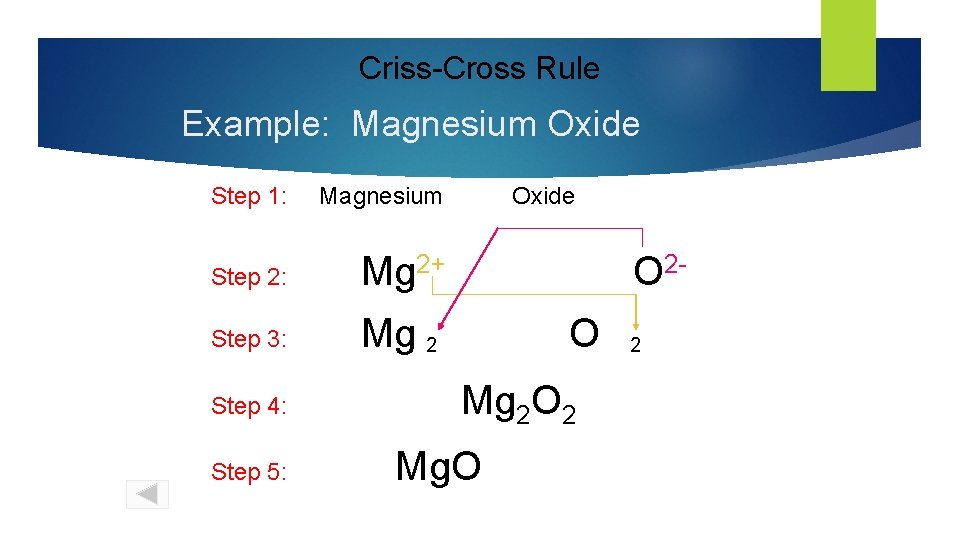
Criss-Cross Rule Example: Magnesium Oxide Step 1: Magnesium Step 2: Mg 2+ Step 3: Mg 2 Step 4: Step 5: Oxide O 2 O Mg 2 O 2 Mg. O 2

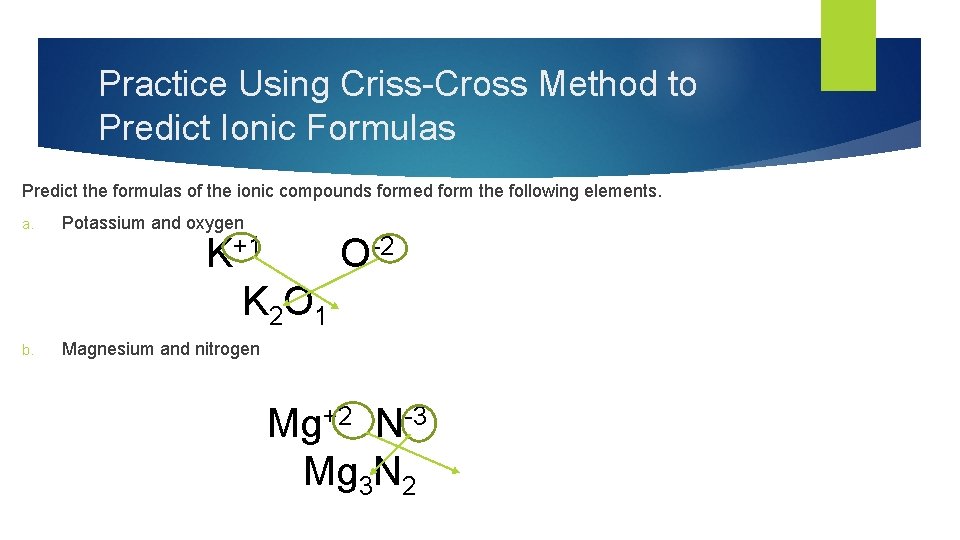
Practice Using Criss-Cross Method to Predict Ionic Formulas Predict the formulas of the ionic compounds formed form the following elements. a. Potassium and oxygen b. Magnesium and nitrogen K+1 O-2 K 2 O 1 Mg+2 N-3 Mg 3 N 2

21 Learning Check What is the correct formula for each of the following? A. Copper(I) nitride 1) Cu. N 2) Cu. N 3 3) Cu 3 N B. Lead(IV) oxide 1) Pb. O 2 2) Pb. O 3) Pb 2 O 4

Summary Steps to writing formulas of binary ionic compounds given the name 1. Write down the Chemical Symbols for each element 2. Determine if the metal is a transition metal. (If a roman numeral is used, it is a transition metal) 3. Using oxidation numbers, determine how many of each element are needed. 4. Write the final formula Ex: Aluminum nitride Aluminum oxide Gold (I) oxide 22