Aim How to write and balance half reactions

Aim: How to write and balance half reactions • Do Now: Identify the following reactions as either redox or nonredox using oxidation numbers as evidence. a. 2 H 2 O → 2 H 2 + O 2 b. CH 4 + 2 O 2 → 2 H 2 O + CO 2 c. HCl + Na. OH → H 2 O + Na. Cl

Identifying if a reaction is a redox reaction • Explain, in terms of electrons and oxidation numbers, how you can identify if a reaction is a redox reaction or not.

Half Reaction • Half reaction shows the exchange of electrons in a redox reaction • All redox reactions can be divided up into two reactions—an oxidation half-reaction and a reduction half-reaction

Example of a Reduction Half Reaction Fe 3+ + 3 e- Fe 0 When Fe 3+(aq) gains 3 electrons, it produces iron solid Fe(s) * Electrons on the left side, gained in the reaction

Example of an Oxidation Half Reaction Fe 0 Fe 3+ + 3 e*Electrons are the right hand side, loss of electrons in the reaction ** Always add electrons to the side of the reaction that has a more positive charge** When iron solid Fe(s) loses 3 electrons, it produces Fe 3+ (aq)

Consider the incomplete half-reactions below. a. Use oxidation numbers to identify the reactions below as oxidation or reduction. b. Place the correct number of electrons on the appropriate side of the reaction to complete the equation. • I 2 → 2 I– • Cr 2+ → Cr 3+ • Sr → Sr 2+
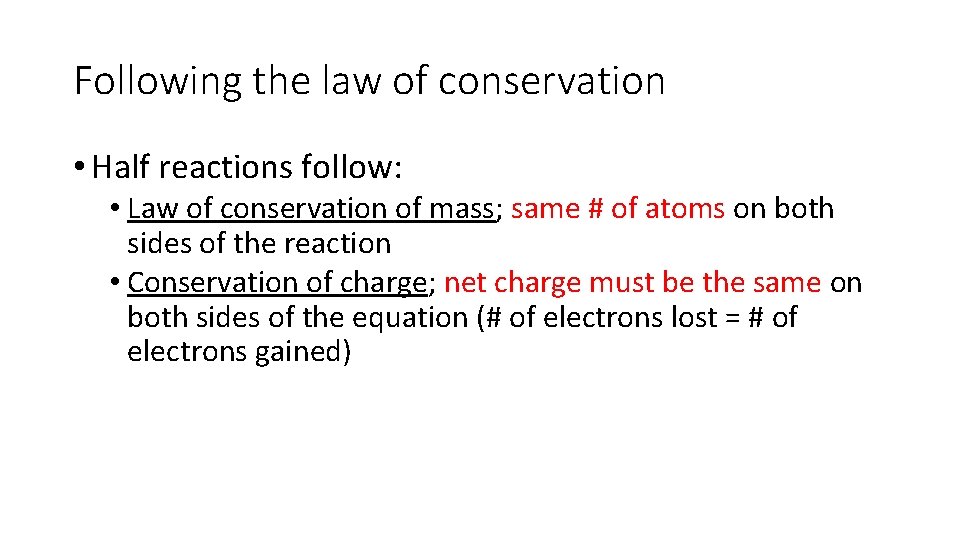
Following the law of conservation • Half reactions follow: • Law of conservation of mass; same # of atoms on both sides of the reaction • Conservation of charge; net charge must be the same on both sides of the equation (# of electrons lost = # of electrons gained)
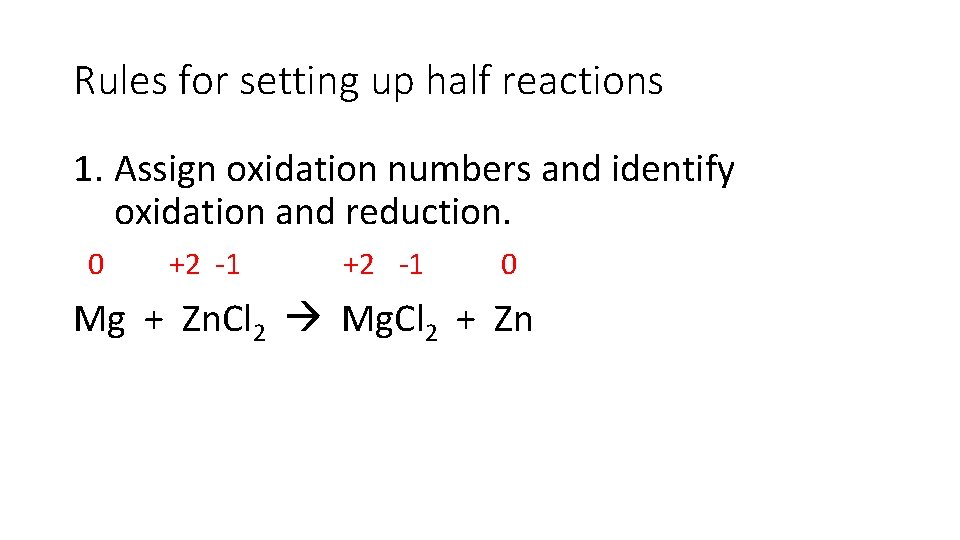
Rules for setting up half reactions 1. Assign oxidation numbers and identify oxidation and reduction. 0 +2 -1 0 Mg + Zn. Cl 2 Mg. Cl 2 + Zn
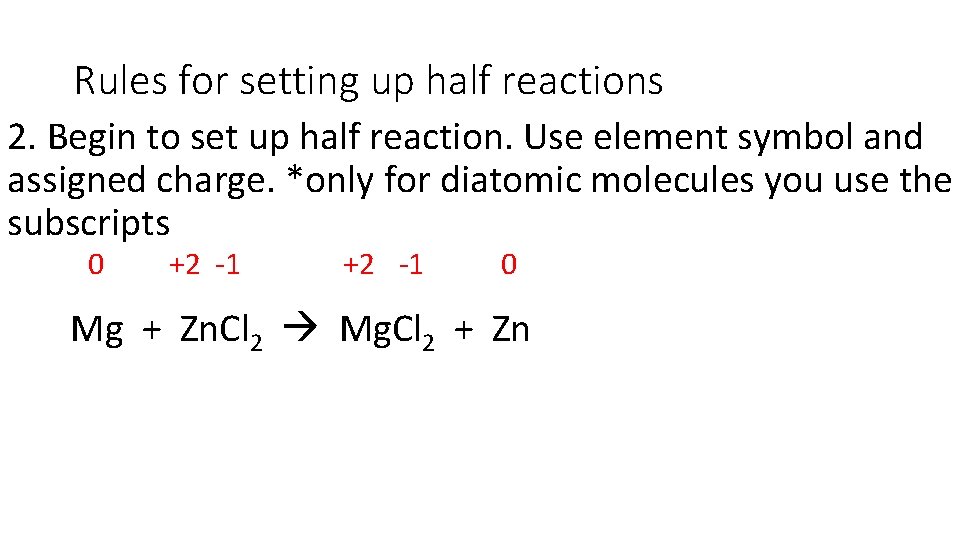
Rules for setting up half reactions 2. Begin to set up half reaction. Use element symbol and assigned charge. *only for diatomic molecules you use the subscripts 0 +2 -1 0 Mg + Zn. Cl 2 Mg. Cl 2 + Zn
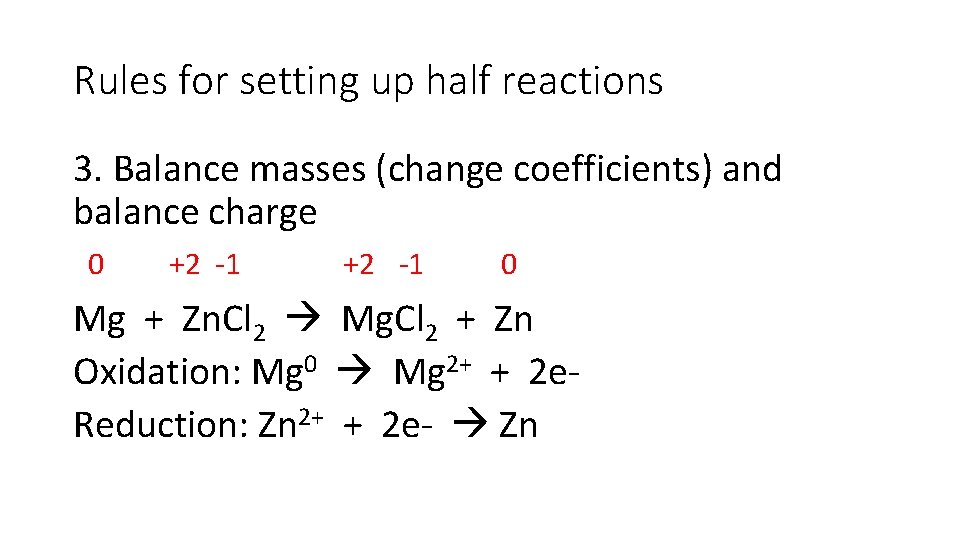
Rules for setting up half reactions 3. Balance masses (change coefficients) and balance charge 0 +2 -1 0 Mg + Zn. Cl 2 Mg. Cl 2 + Zn Oxidation: Mg 0 Mg 2+ + 2 e. Reduction: Zn 2+ + 2 e- Zn

Balancing with Half-Reactions • Balance the following redox reaction using half reactions: Fe + O 2 Fe 2 O 3 • First, assign oxidation numbers • Second, write the oxidation and reduction half reactions. Make sure to balance number of atoms. • Third, multiply each half-reaction to have the same number of electrons.
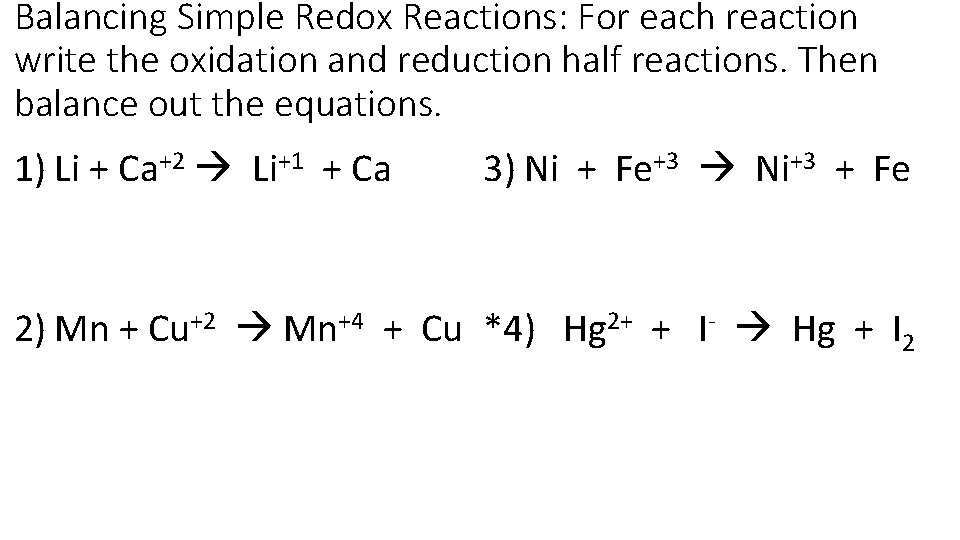
Balancing Simple Redox Reactions: For each reaction write the oxidation and reduction half reactions. Then balance out the equations. 1) Li + Ca+2 Li+1 + Ca 3) Ni + Fe+3 Ni+3 + Fe 2) Mn + Cu+2 Mn+4 + Cu *4) Hg 2+ + I- Hg + I 2
- Slides: 12