Aim How to identify elements based on their

Aim: How to identify elements based on their atomic spectra Do Now: 1. Explain how an electron becomes excited. 2. Write the excited state for the phosphorus atom

What gives gas-filled lights their colors? • An electric current passing through the gas in each glass tube makes the gas glow with its own characteristic color.
The Nature of Light • Light is a part of the electromagnetic spectrum radiant energy composed of gamma rays, X-rays, ultraviolet light, visible light, etc. • The energy of the electromagnetic spectrum moves through space as waves

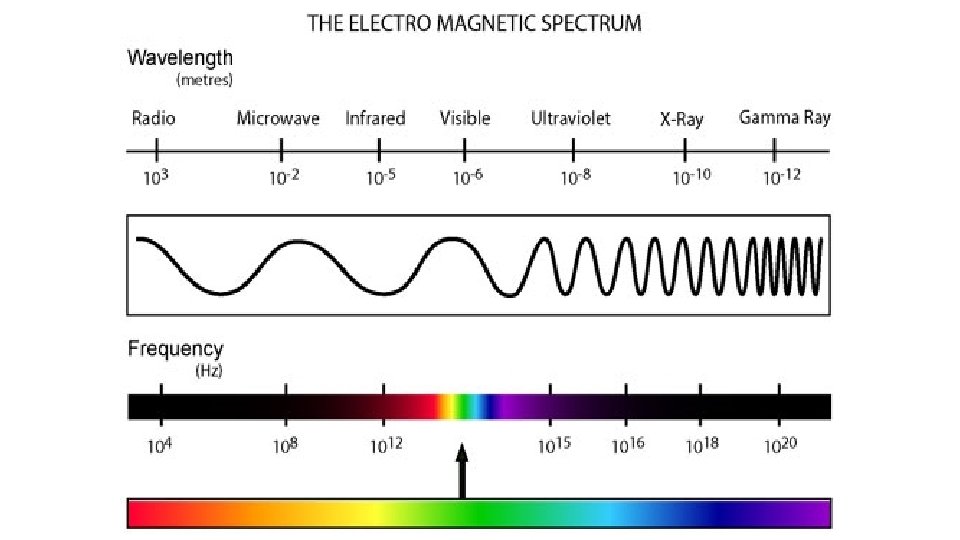
Nature of Light • Sunlight consists of light with a continuous range of wavelengths and frequencies. – When sunlight passes through a prism, the different frequencies separate into a spectrum of colors. – In the visible spectrum, red light has the longest wavelength and the lowest frequency.
Atomic Spectra • The energy absorbed by the electron to go from the ground state to the excited state is equal to energy released or emitted by the electron upon returning to ground state. • The excitation fallback theory explains the visible emission (bright line) spectrum of element

Emission Spectrum • A prism separates light into the colors it contains. White light produces a rainbow of colors.
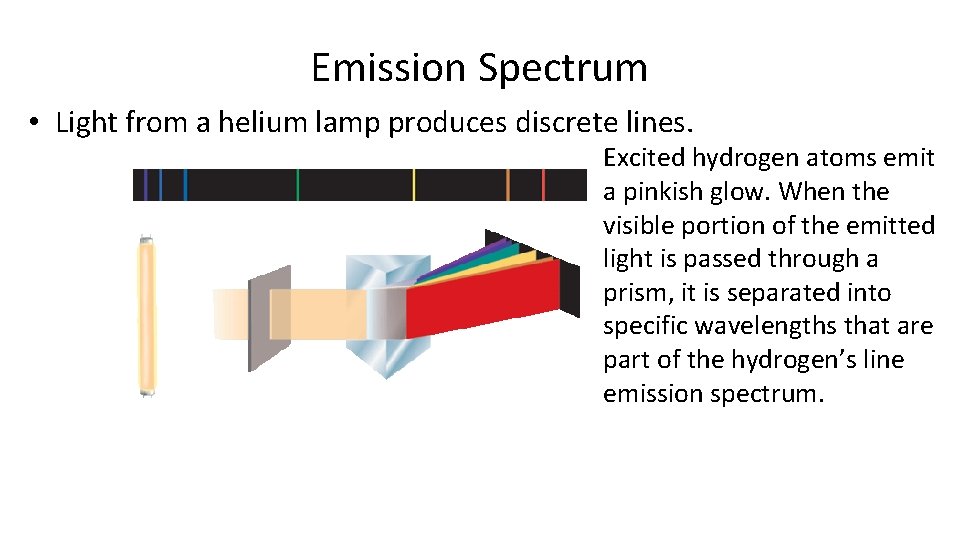
Emission Spectrum • Light from a helium lamp produces discrete lines. Excited hydrogen atoms emit a pinkish glow. When the visible portion of the emitted light is passed through a prism, it is separated into specific wavelengths that are part of the hydrogen’s line emission spectrum.
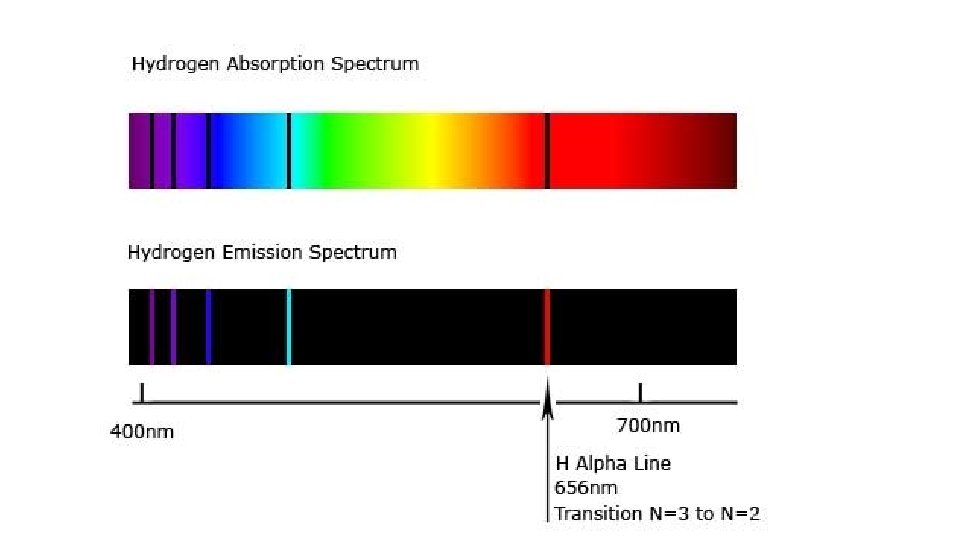
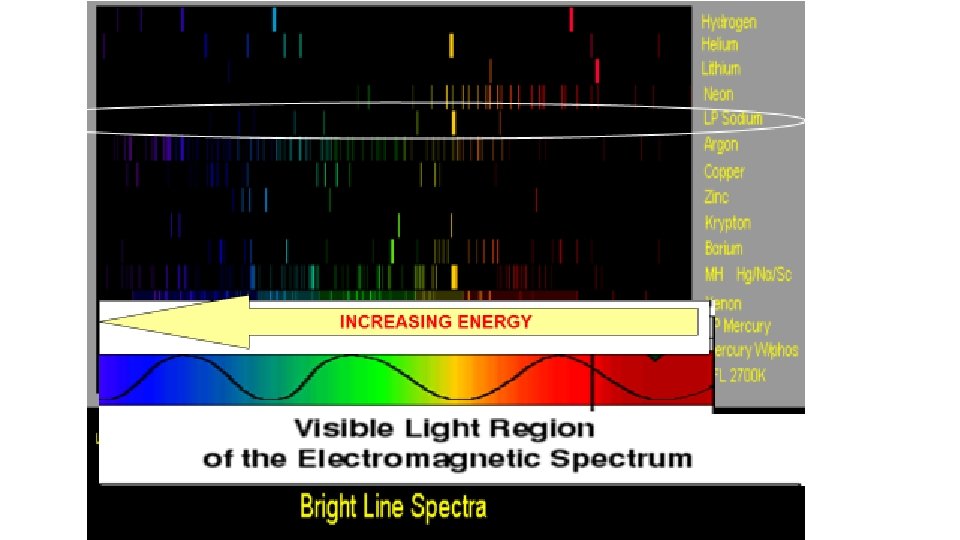
Emission Spectrum • The wavelengths of the spectral lines are characteristic of the element, and they make up the atomic emission spectrum of the element. • No two elements have the same emission spectrum. – The fingerprints of an element.
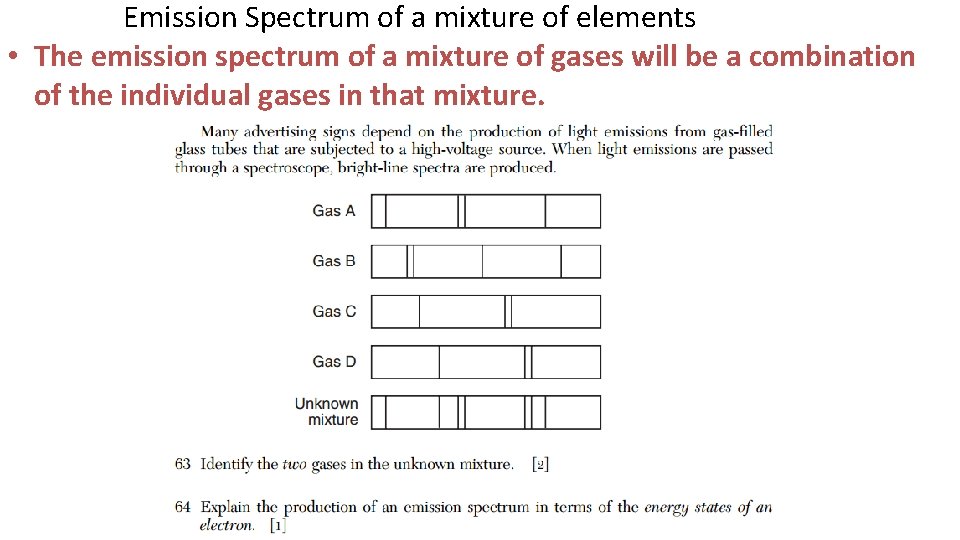
Emission Spectrum of a mixture of elements • The emission spectrum of a mixture of gases will be a combination of the individual gases in that mixture.
- Slides: 12