Aim How to graphically represent the energy change





- Slides: 23

Aim: How to graphically represent the energy change in a system? Do Now: Answer the following questions using Table I 1. Explain, in terms of heat, what happens during the following reaction: N 2(g) + 2 O 2(g) 2 NO 2(g) Which has more energy, the reactants or products? 2. What is the heat of reaction for the following reaction: 2 NH 3(g) N 2(g) + 3 H 2(g) Rewrite the equation so that heat is part of the equation 3. Given the reaction: 4 Al(s) + 3 O 2(g) 2 Al 2 O 3(s) Calculate how much heat is released when 5 moles of aluminum completely reacts with oxygen.

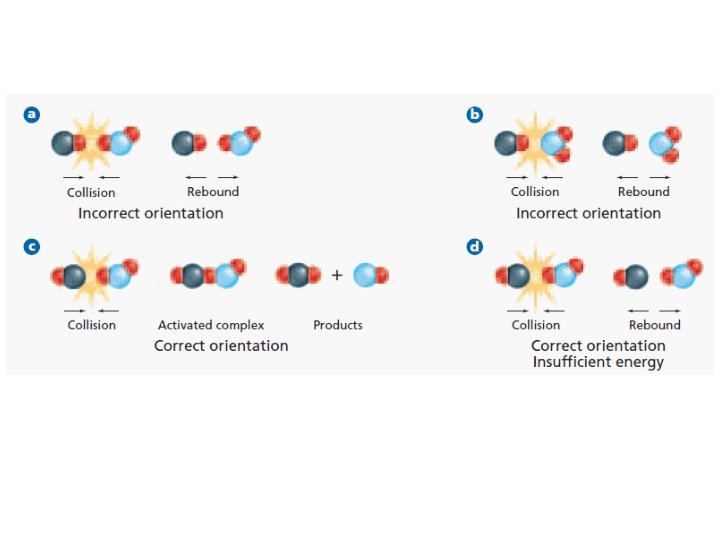
Collision Theory For a reaction to happen, the particles must have effective collisions; – particles must collide with enough energy to overcome the activation energy • Activation Energy is smallest amount of energy needed to start a reaction – Particles must collide at the correct angles.


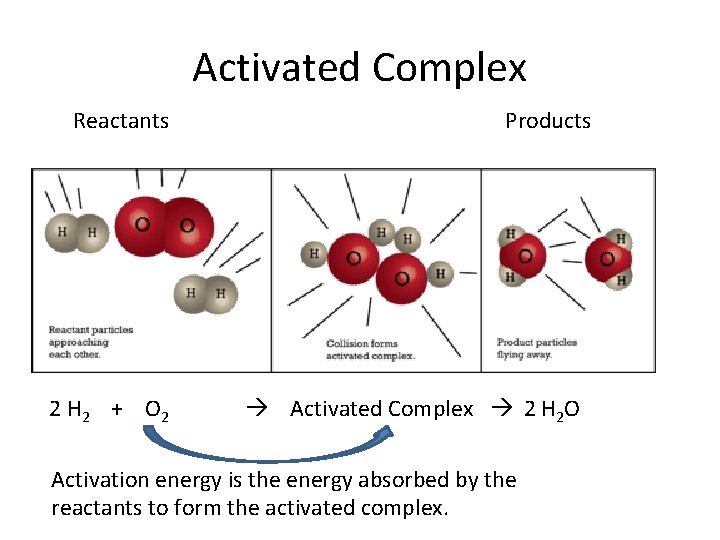
Activation Energy and Activated Complex • ALL REACTIONS need to absorb energy to overcome the activation energy. • When the reactants have absorbed enough energy, they form an activated complex (transition state between reactants and products). • The activated complex has more stored energy (potential energy) than the starting reactants and final products

Activated Complex Reactants 2 H 2 + O 2 Products Activated Complex 2 H 2 O Activation energy is the energy absorbed by the reactants to form the activated complex.

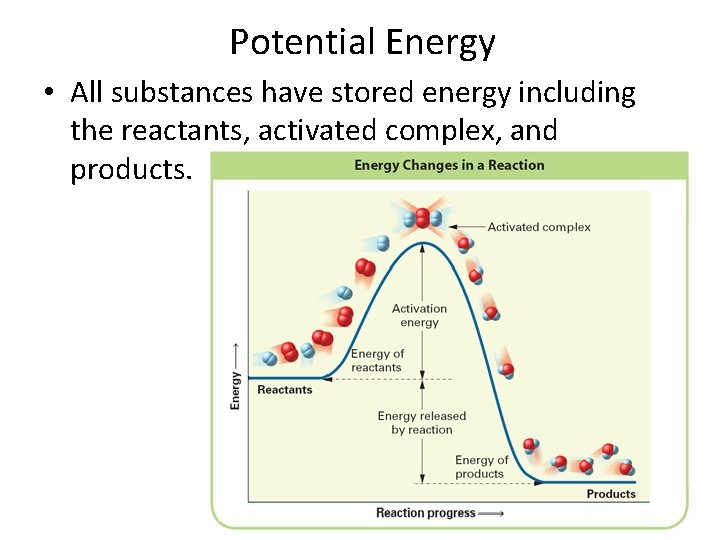
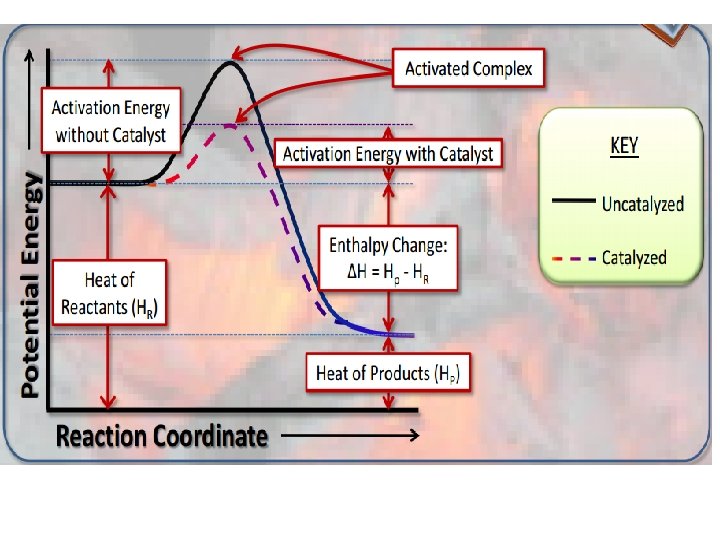
What information can you determine from a potential energy diagram A potential energy diagram can be defined as a diagram showing the relative potential energies of reactants, activated complex(transition state), and products as a reaction progresses with time. • The PE of the reactants • The PE of the products • The PE of the activated complex • The activation energy • The heat of reaction ΔH • If the reaction is exothermic or endothermic

Potential Energy • All substances have stored energy including the reactants, activated complex, and products.

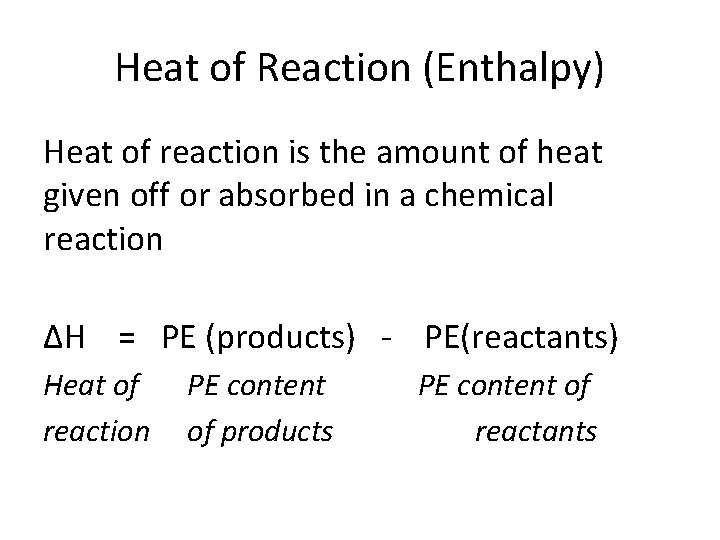
Heat of Reaction (Enthalpy) Heat of reaction is the amount of heat given off or absorbed in a chemical reaction ΔH = PE (products) - PE(reactants) Heat of reaction PE content of products PE content of reactants

Sketching Potential Energy Diagrams When sketching or analyzing a PE diagram, keep in mind: – Activated complex has more energy than both the reactants and products (the reactants absorb energy to form activated complex) – In an endothermic reaction, the products have more energy than the reactants – In an exothermic reaction, the products has less energy than the reactants

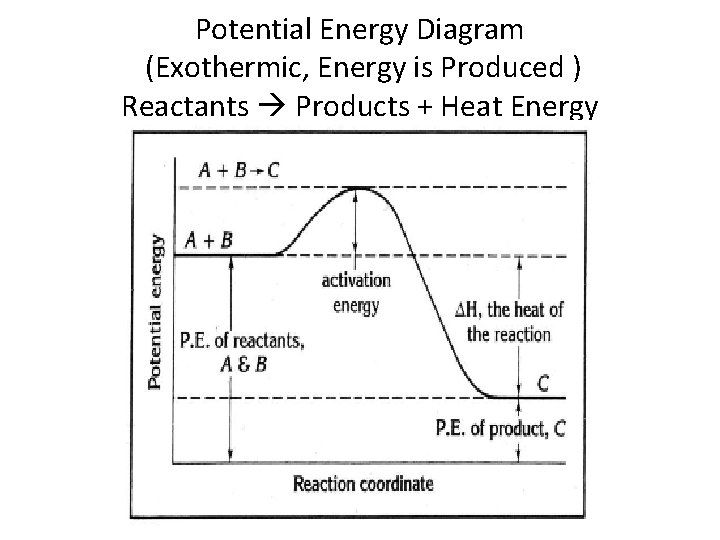
Potential Energy Diagram (Exothermic, Energy is Produced ) Reactants Products + Heat Energy

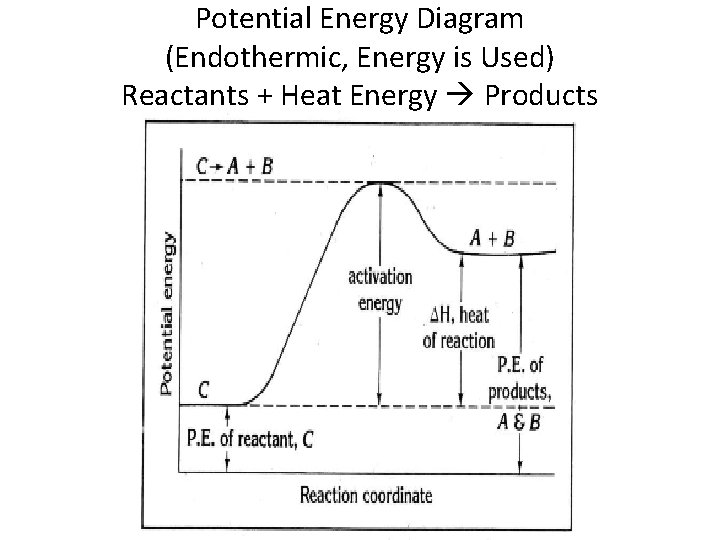
Potential Energy Diagram (Endothermic, Energy is Used) Reactants + Heat Energy Products

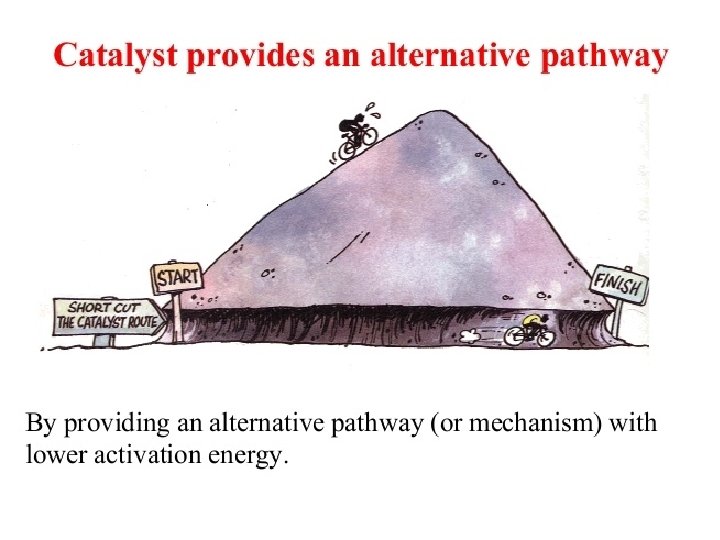
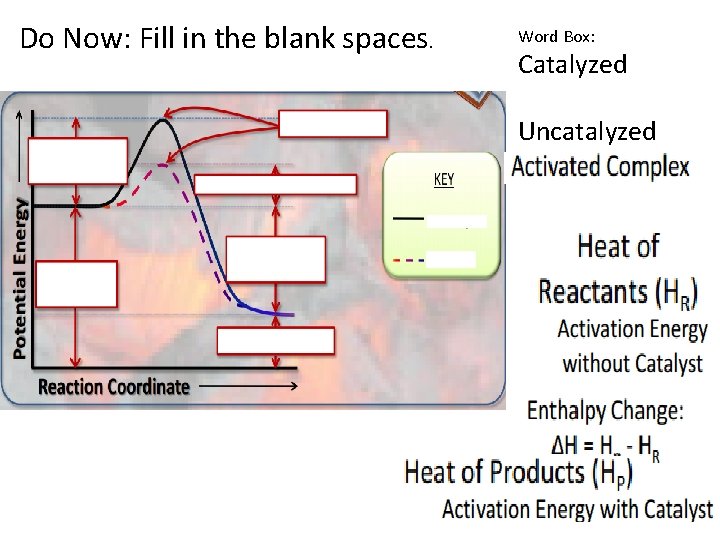
Addition of Catalyst • A catalyst speeds up a reaction by lowering the amount of energy needed to start the reaction so that a reaction can happen more easily by providing an alternate pathway.


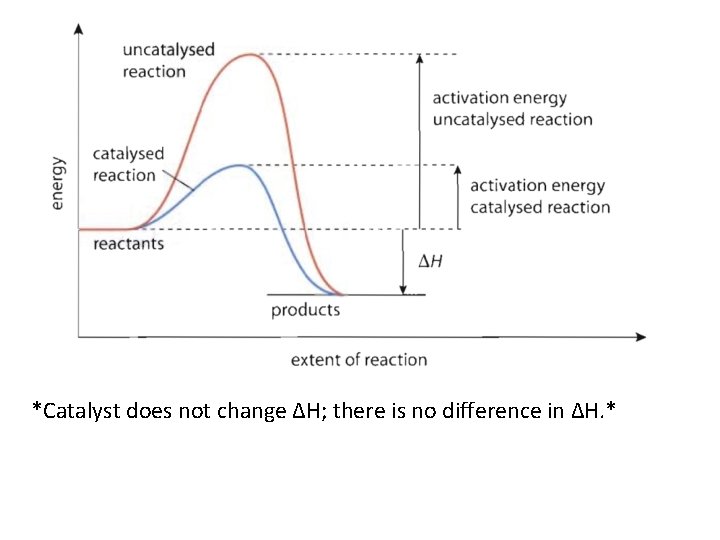
*Catalyst does not change ΔH; there is no difference in ΔH. *

Reading in the forward and reverse direction • A PE diagram can be read in the forward direction ( ) or the reverse direction ( ). In the forward direction, the reactants are on the left, and the products are on the right. In the reverse direction, the reactants are on the right, and the products are on the left.

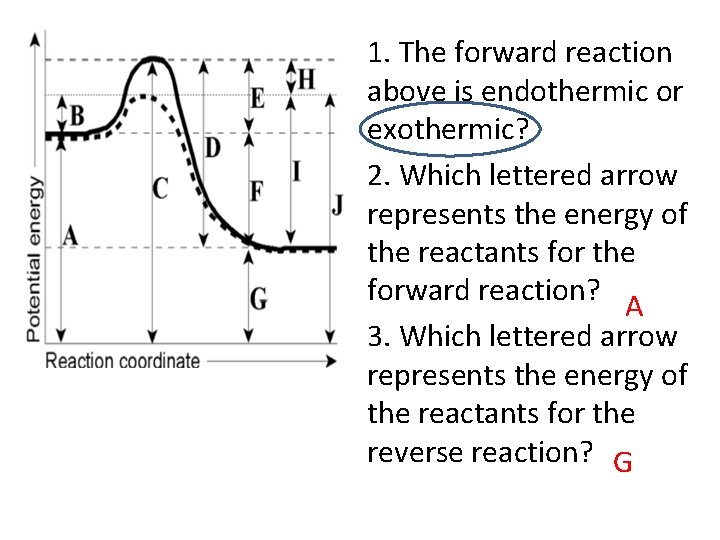
1. The forward reaction above is endothermic or exothermic? 2. Which lettered arrow represents the energy of the reactants for the forward reaction? A 3. Which lettered arrow represents the energy of the reactants for the reverse reaction? G

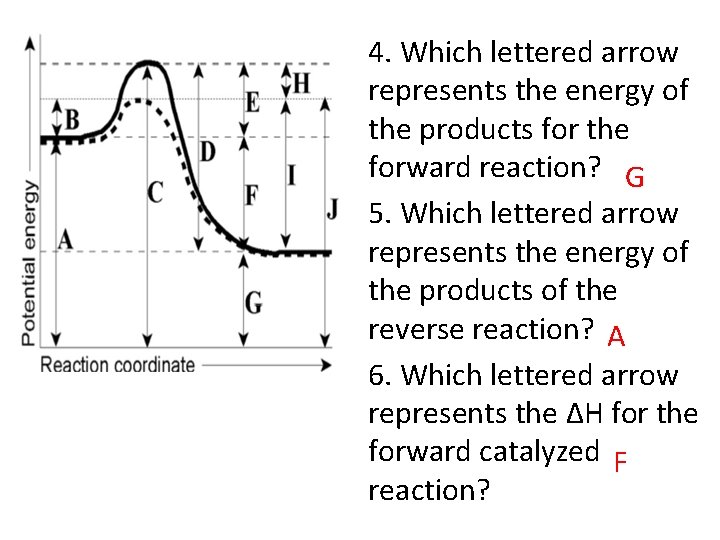
4. Which lettered arrow represents the energy of the products for the forward reaction? G 5. Which lettered arrow represents the energy of the products of the reverse reaction? A 6. Which lettered arrow represents the ΔH for the forward catalyzed F reaction?

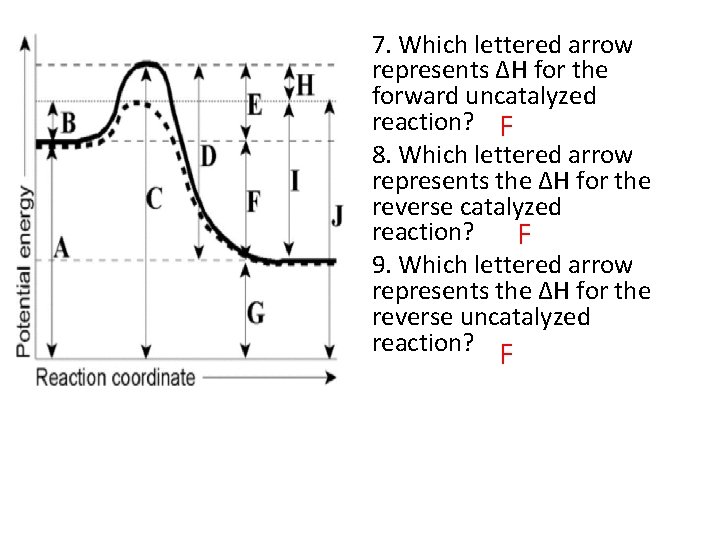
7. Which lettered arrow represents ΔH for the forward uncatalyzed reaction? F 8. Which lettered arrow represents the ΔH for the reverse catalyzed reaction? F 9. Which lettered arrow represents the ΔH for the reverse uncatalyzed reaction? F

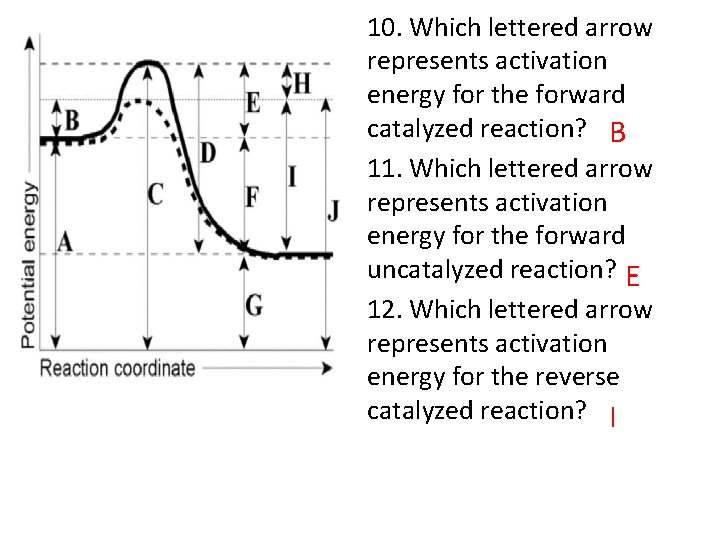
10. Which lettered arrow represents activation energy for the forward catalyzed reaction? B 11. Which lettered arrow represents activation energy for the forward uncatalyzed reaction? E 12. Which lettered arrow represents activation energy for the reverse catalyzed reaction? I

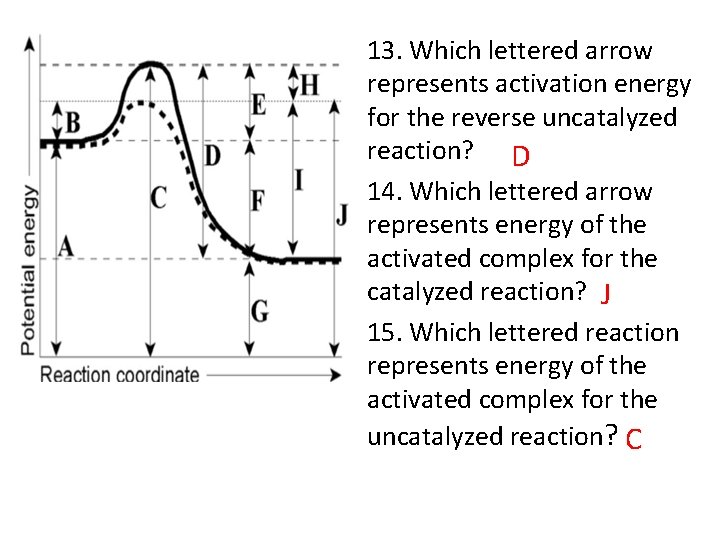
13. Which lettered arrow represents activation energy for the reverse uncatalyzed reaction? D 14. Which lettered arrow represents energy of the activated complex for the catalyzed reaction? J 15. Which lettered reaction represents energy of the activated complex for the uncatalyzed reaction? C

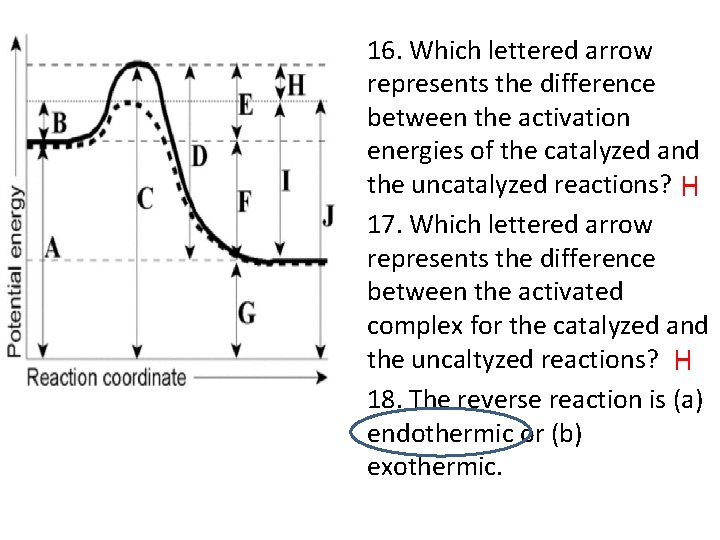
16. Which lettered arrow represents the difference between the activation energies of the catalyzed and the uncatalyzed reactions? H 17. Which lettered arrow represents the difference between the activated complex for the catalyzed and the uncaltyzed reactions? H 18. The reverse reaction is (a) endothermic or (b) exothermic.

Do Now: Fill in the blank spaces. Word Box: Catalyzed Uncatalyzed
