AIM HOW TO DETERMINE IONIZATION ENERGY AND ELECTRONEGATIVITY













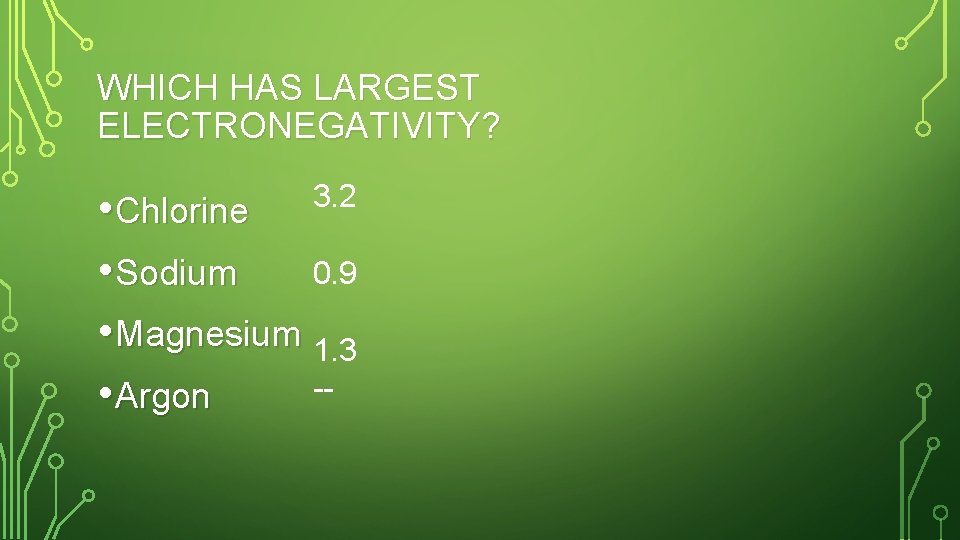






- Slides: 32

AIM: HOW TO DETERMINE IONIZATION ENERGY AND ELECTRONEGATIVITY OF ELEMENTS DO NOW: SELECT THE GREATEST RADIUS FOR EACH ELEMENT SET 1: NA, K, NA+, K+ SET 2: NA+, K+, MG 2+, CA 2+ SET 3: F, F-, NA+

REVIEW OF IONS • Atoms gain or lose electrons to have a stable electron configuration (full outer shell) like the noble gases. • Positive and negative ions form when electrons are transferred between atoms. (Na+1, Ca+2) • Metals tend to lose electrons, forming positive ions; nonmetals tend to gain those electrons forming negative ions (N-3, O-2, Cl-1)

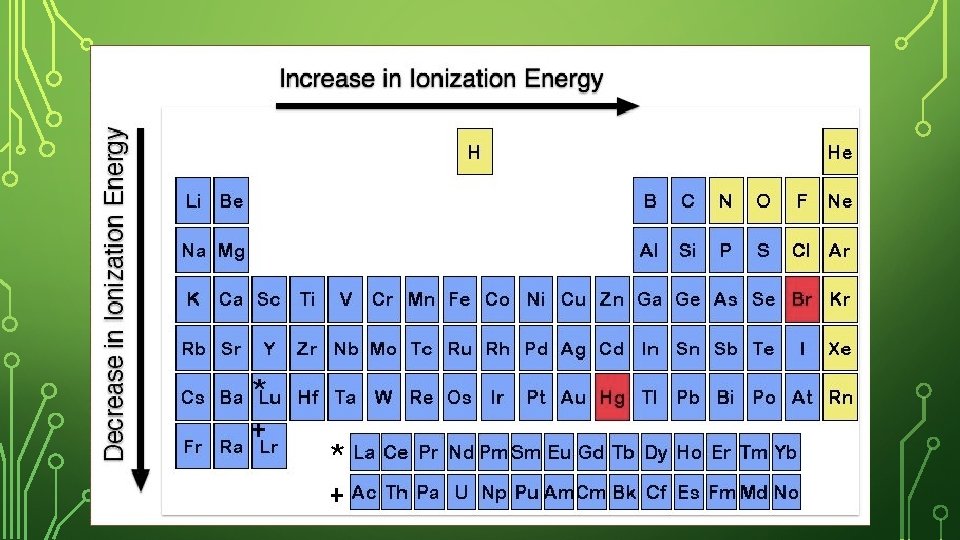
IONIZATION ENERGY • The energy required to remove an electron from an atom. • The larger the ionization energy value the harder to lose an electron (the stronger the atom is holding on to its electron)!

FIRST IONIZATION ENERGY • The first ionization energy is the energy required to remove the first electron from its atom. (Table S)

SECOND AND THIRD IONIZATION ENERGY • The second ionization energy is the energy required to remove an electron from an ion with a 1+ charge. • The third ionization energy is the energy required to remove an electron from an ion with a 2+ charge.

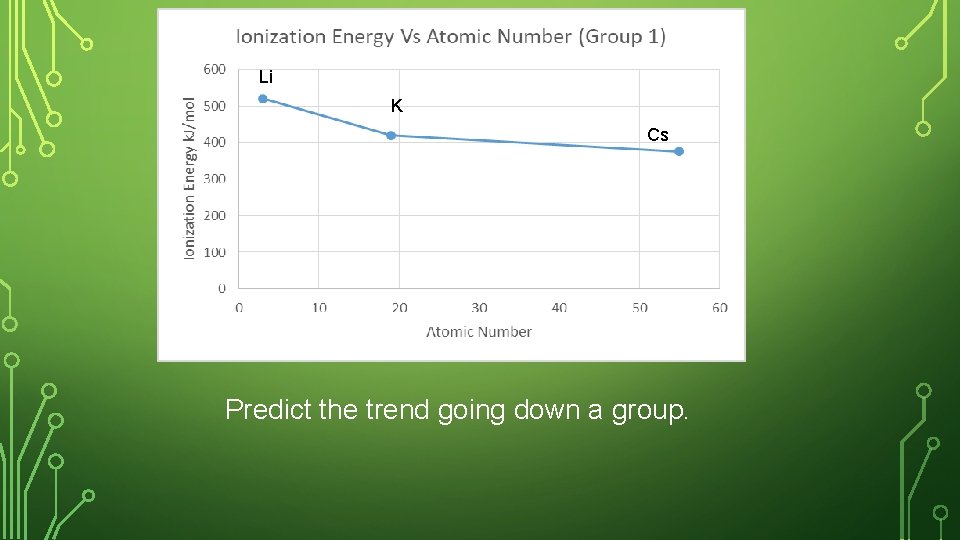
SKETCH IN NOTEBOOK • Create a graph in your notebook. Label the y- axis as First Ionization Energy, and the x-axis as atomic number. • Plot the ionization energy and the atomic number for the following elements. Label your point with the element symbol • Lithium, potassium, cesium • Where are these 3 elements placed in the periodic table?

Li K Cs Predict the trend going down a group.

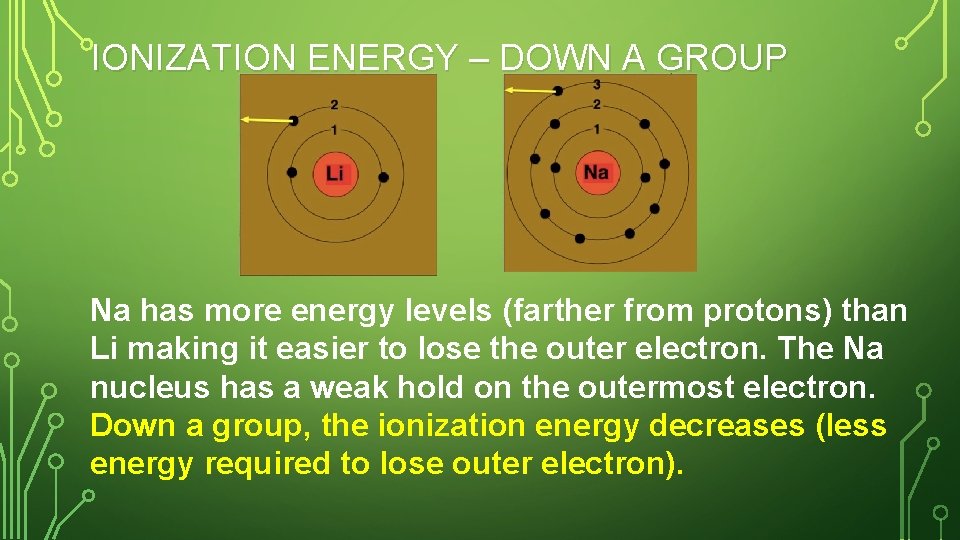
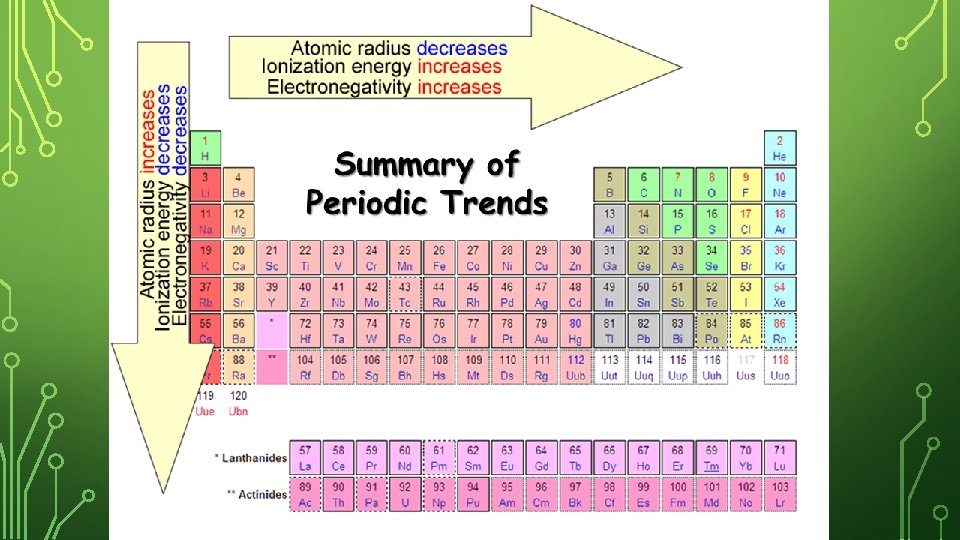
IONIZATION ENERGY – DOWN A GROUP Na has more energy levels (farther from protons) than Li making it easier to lose the outer electron. The Na nucleus has a weak hold on the outermost electron. Down a group, the ionization energy decreases (less energy required to lose outer electron).

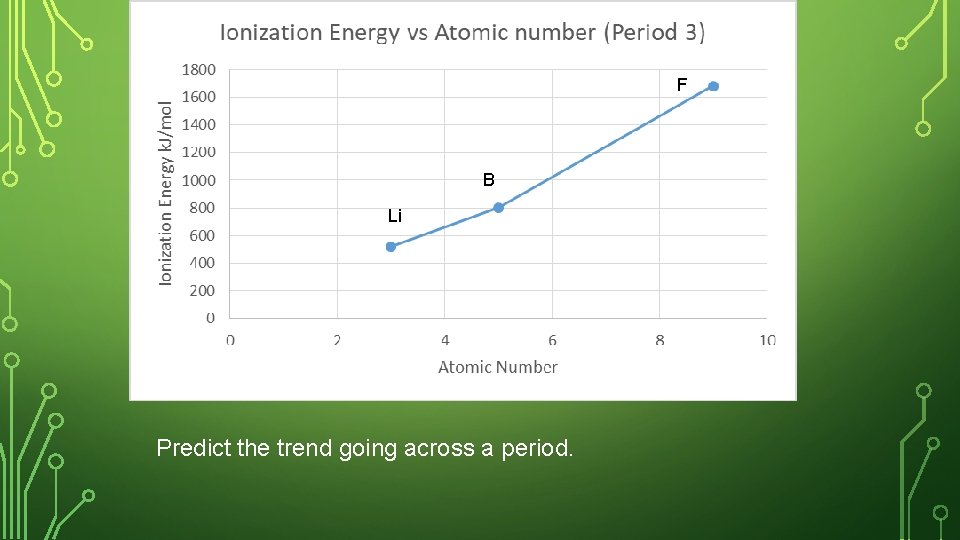
SKETCH IN NOTEBOOK • Create a graph in your notebook. Label the y- axis as First Ionization Energy, and the x-axis as atomic number. • Plot the ionization energy and the atomic number for the following elements. Label your point with the element symbol • Lithium, boron, fluorine • Where are these 3 elements placed in the periodic table?

F B Li Predict the trend going across a period.

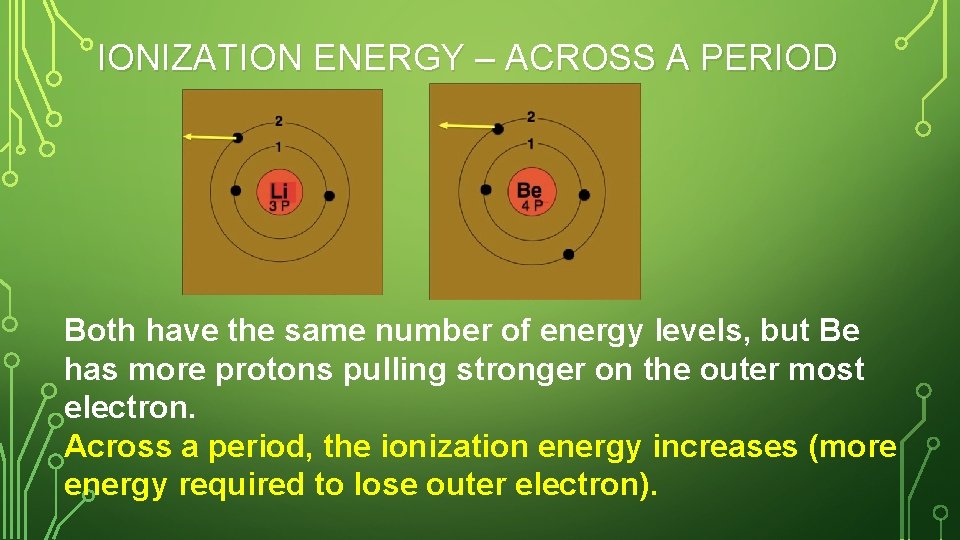
IONIZATION ENERGY – ACROSS A PERIOD Both have the same number of energy levels, but Be has more protons pulling stronger on the outer most electron. Across a period, the ionization energy increases (more energy required to lose outer electron).


WHICH HAS LOWEST IONIZATION ENERGY? 1251 k. J/mol • Chlorine 496 k. J/mol • Sodium • Magnesium 738 k. J/mol • Argon 1521 k. J/mol

AIM: HOW TO DETERMINE IONIZATION ENERGY AND ELECTRONEGATIVITY OF ELEMENTS (CONTINUED) Do Now: Answer in your notebook and explain your answer. Which of the following requires the least amount of energy to remove the outermost electron? a. b. c. Sr Sn Te

ELECTRONEGATIVITY • The ability of an atom to attract electrons when the atom is in a compound. (Table S) • The ability of an atom to steal an electron from another atom (electron thieves).

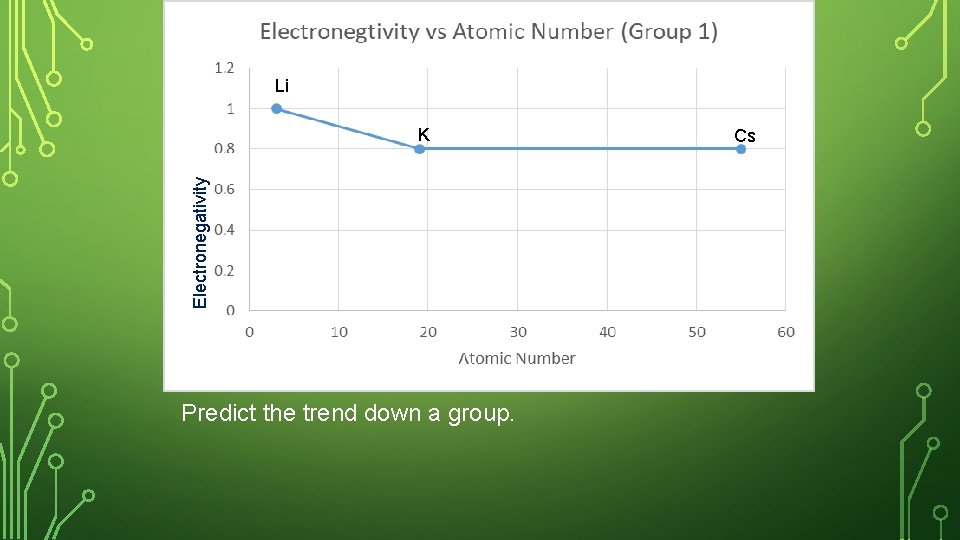
SKETCH IN NOTEBOOK • Create a graph in your notebook. Label the y- axis as Electronegativity, and the x-axis as atomic number. • Plot the ionization energy and the atomic number for the following elements. Label your point with the element symbol • Lithium, potassium, cesium • Where are these 3 elements placed in the periodic table?

Li Electronegativity K Predict the trend down a group. Cs

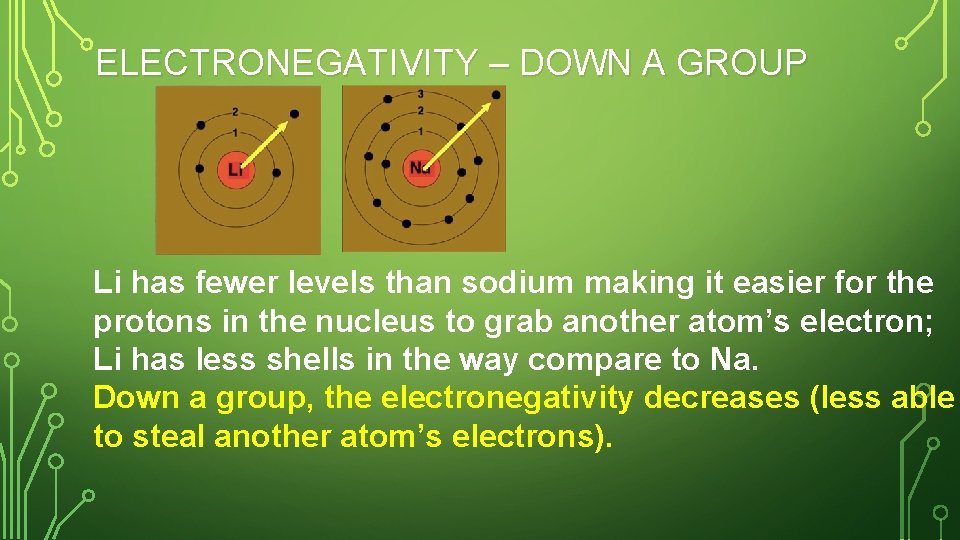
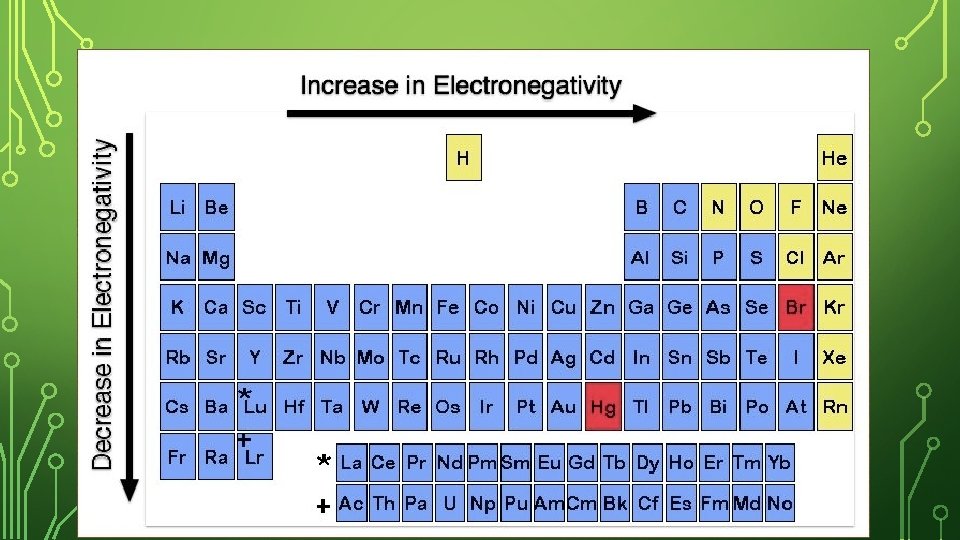
ELECTRONEGATIVITY – DOWN A GROUP Li has fewer levels than sodium making it easier for the protons in the nucleus to grab another atom’s electron; Li has less shells in the way compare to Na. Down a group, the electronegativity decreases (less able to steal another atom’s electrons).

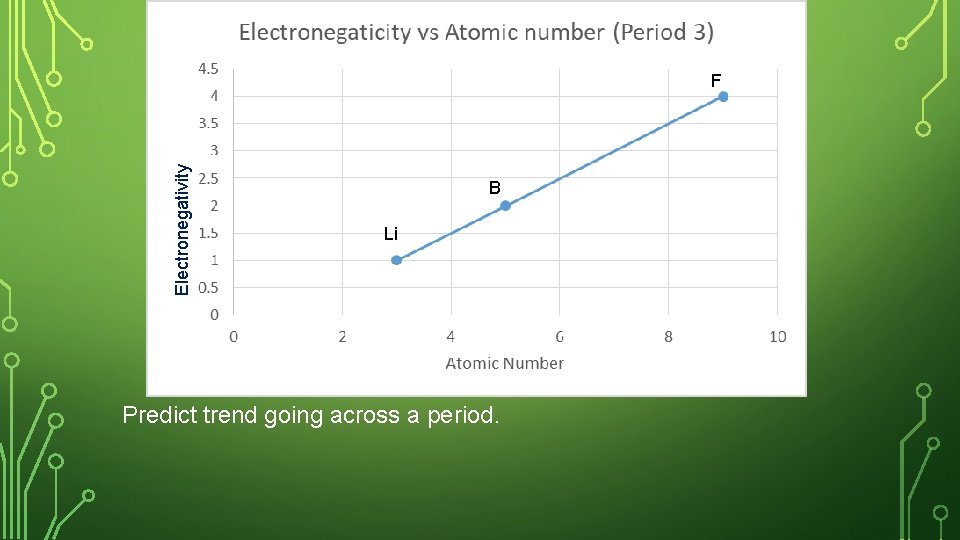
SKETCH IN NOTEBOOK • Create a graph in your notebook. Label the y- axis as Electronegativity, and the x-axis as atomic number. • Plot the ionization energy and the atomic number for the following elements. Label your point with the element symbol • Lithium, boron, and fluorine • Where are these 3 elements placed in the periodic table?

Electronegativity F B Li Predict trend going across a period.

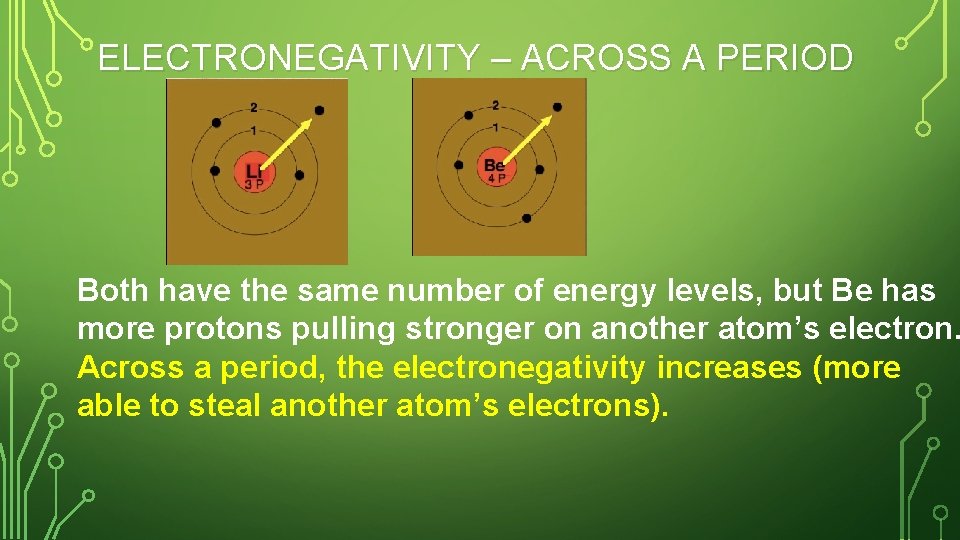
ELECTRONEGATIVITY – ACROSS A PERIOD Both have the same number of energy levels, but Be has more protons pulling stronger on another atom’s electron. Across a period, the electronegativity increases (more able to steal another atom’s electrons).


ELECTRONEGATIVITY OF NOBLE GASES • What are the electronegativity values of Neon, Argon, and Krypton? • Noble gases are not electronegative • Why do you think, in terms of valence electrons, why the noble gases are not electronegative? • Noble gases already have a stable electron configuration (full outer shell); they do not need to steal electrons from another atom to become stable
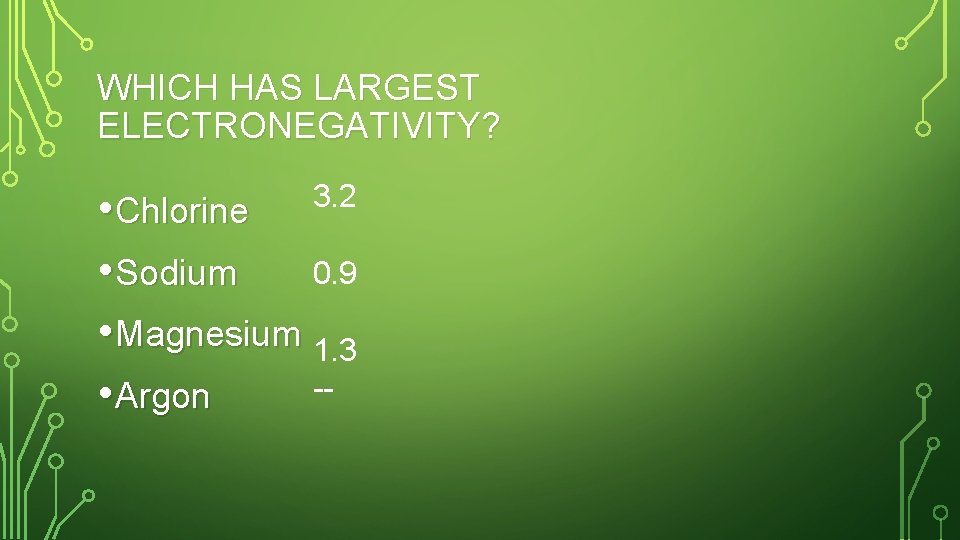
WHICH HAS LARGEST ELECTRONEGATIVITY? 3. 2 • Chlorine 0. 9 • Sodium • Magnesium 1. 3 - • Argon

METALLIC CHARACTER • The metallic character of an element can be defined as how readily an atom can lose an electron. • Metallic characteristics decrease from left to right across a period, and increase down a group.


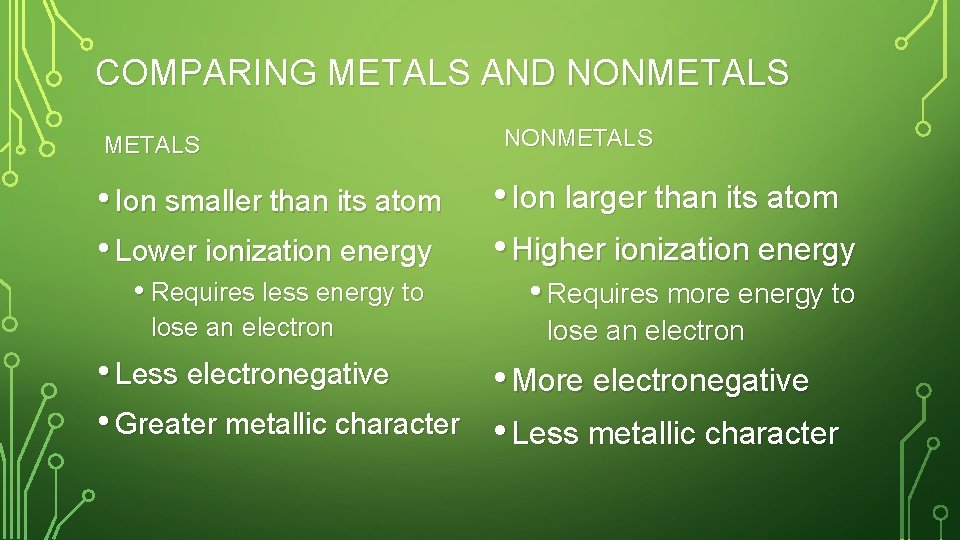
COMPARE TREND IN METAL AND NONMETAL • For each type list: • • Is the ion smaller or bigger than the atom Is ionization energy high or low Is electronegativity high or low And is metallic character high or low

COMPARING METALS AND NONMETALS • Ion smaller than its atom • Lower ionization energy • Requires less energy to lose an electron • Less electronegative • Greater metallic character NONMETALS • Ion larger than its atom • Higher ionization energy • Requires more energy to lose an electron • More electronegative • Less metallic character

REGENTS QUESTION 1 Which statement describes the general trends in electronegativity and metallic properties as the elements in Period 2 are considered in order of increasing atomic number? (1) Both electronegativity and metallic properties decrease. (2) Both electronegativity and metallic properties increase. (3) Electronegativity decreases and metallic properties increase. (4) Electronegativity increases and metallic properties decrease Choice 4: electron negativity increases (heading to Fluorine)metallic character decreases you are leaving the metals

REGENTS QUESTION 2 Which statement describes the general trends in electronegativity and first ionization energy as the elements in Period 3 are considered in order from Na to Cl? (1) Electronegativity increases, and first ionization energy decreases. (2) Electronegativity decreases, and first ionization energy decreases. (3) Electronegativity and first ionization energy both increase. (4) Electronegativity firstand ionization energyincrease both Choice 3: Both ionizationand energy electronegativity decrease. from left to right.

REGENTS QUESTION 3 The amount of energy required to remove the outermost electron from a gaseous atom in the ground state is known as (1) first ionization energy (2) activation energy (3) conductivity (4) electronegativity Choice 1: definition of ionization energy

REGENTS QUESTION 4 Which term represents the attraction one atom has for the electrons in a bond from another atom? (1) electronegativity (2) electrical conductivity (3) first ionization energy (4) mechanical energy Choice 1: Definition of electronegativity is the ability to attract an electron from another atom