Aim How to determine if a solution is



![Measuring p. H • If [H+] is 1. 0 x 10 -4, p. H Measuring p. H • If [H+] is 1. 0 x 10 -4, p. H](https://slidetodoc.com/presentation_image_h/10fcc5ed6a8bc0b7dd24654c80ba401d/image-4.jpg)









- Slides: 18

Aim: How to determine if a solution is acidic, basic, or neutral

Acidic, Basic, and Neutral Solutions • Acidic solutions (acid in water (H 2 O)) have more H+ (H 3 O+) than OH-. • Basic solutions have more OH- than H+ (H 3 O+). • H 2 O (water alone) has the same number of H+ as OHions.

The p. H concept • The p. H scale is used to measure the hydrogen-ion concentration [H+]. • p. H = -log[H+] • Acidic solutions have a p. H< 7, a neutral solution has a p. H=7, and a basic solution has a p. H>7.
![Measuring p H If H is 1 0 x 10 4 p H Measuring p. H • If [H+] is 1. 0 x 10 -4, p. H](https://slidetodoc.com/presentation_image_h/10fcc5ed6a8bc0b7dd24654c80ba401d/image-4.jpg)
Measuring p. H • If [H+] is 1. 0 x 10 -4, p. H is 4. The exponent gives you the p. H of a solution. • At p. H 4, [H+] = 1 x 10 -4 M = 0. 0001 M. • A solution with p. H 3, [H+] = 1 x 10 -3 M = 0. 001 M • When the p. H decreases by 1 unit the concentration of hydrogen ions increases by 10 x.

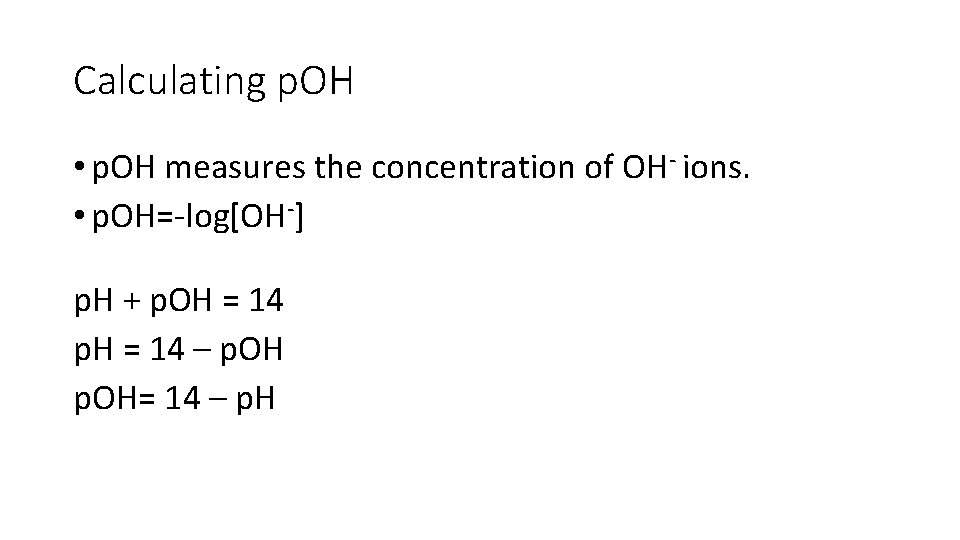
Calculating p. OH • p. OH measures the concentration of OH- ions. • p. OH=-log[OH-] p. H + p. OH = 14 p. H = 14 – p. OH= 14 – p. H

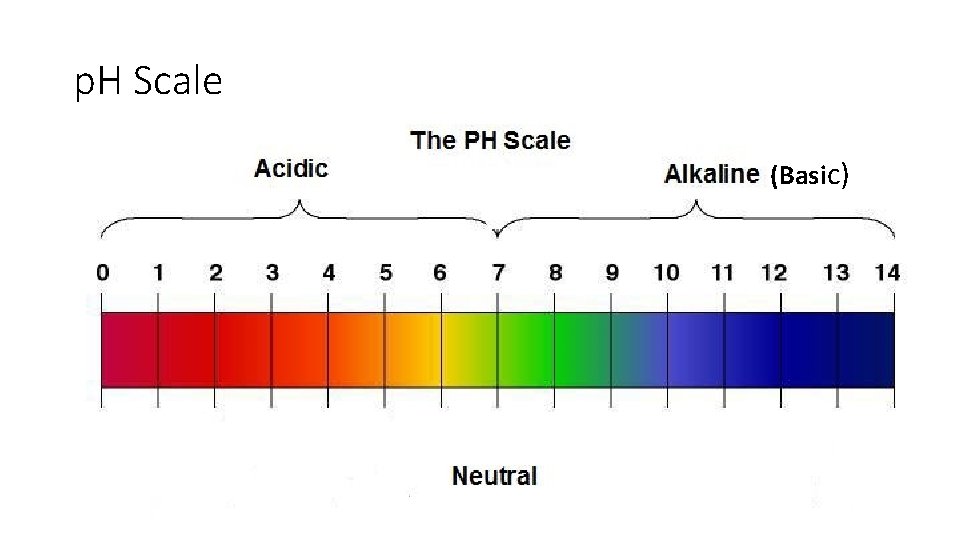
p. H Scale (Basic)

Do Now • An acidic solution can have a p. H of 1. 7 2. 10 3. 3 4. 14 • An aqueous solution that has a hydrogen ion concentration of 1. 0 x 10 -8 mole per liter (M) has a p. H of 1. 6, which is basic 2. 6, which is acidic 3. 8, which is basic 4. 8, which is acidic • What is the p. H of 0. 00001 molar HCl solution? 1. 1 2. 9 3. 5 4. 4

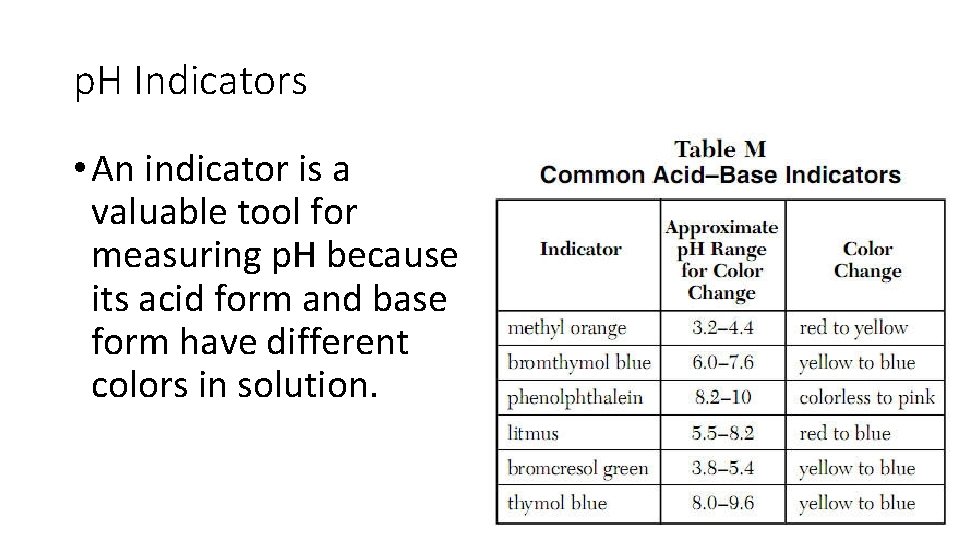
p. H Indicators • An indicator is a valuable tool for measuring p. H because its acid form and base form have different colors in solution.

Methyl orange • Red p. H 3. 2 or less • Yellow p. H 4. 4 or more • Orange p. H 3. 2 to 4. 4

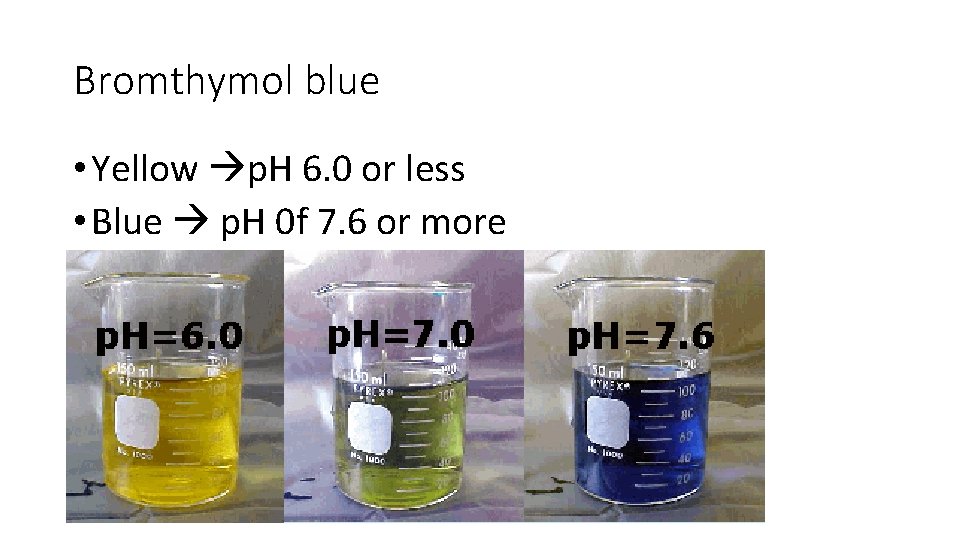
Bromthymol blue • Yellow p. H 6. 0 or less • Blue p. H 0 f 7. 6 or more

Phenolphthalein • Colorless p. H 8. 2 or less • Pink p. H 9 or more

Litmus • Turns blue litmus paper red for acidic solutions • Turns red litmus paper blue for basic solution

Bromcresol green • Yellow p. H 3. 8 or less • Blue p. H 5. 4 or more

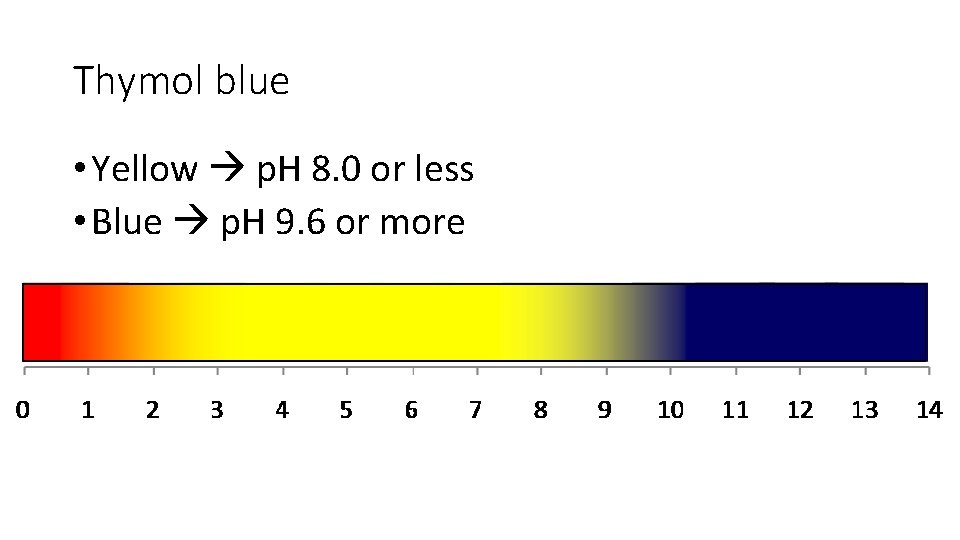
Thymol blue • Yellow p. H 8. 0 or less • Blue p. H 9. 6 or more

Using more than one indicator • If you put bromcresol green in a solution and the solution turns blue, the p. H is 5. 4 or more. • If you put bromthymol blue in another test tube with the same solution and it turns yellow, it shows the p. H is 6. 0 or less. • Conclusion: the solution has a p. H between 5. 4 and 6. 0

Question • A solution turns yellow with thymol blue and blue with bromthymol blue. What is the p. H?

Question • If an aqueous solution turns blue litmus red, which relationship exists between the hydronium ion and the hydroxide ion concentration? 1. [H 3 O+] > [OH-] 2. [H 3 O+] < [OH-] 3. [H 3 O+] = [OH-]= 10 -7 4. [H 3 O+] = [OH-]= 10 -14

Summary ACIDS BASES • p. H below 7 • Litmus red • Phelphthalein clear • Bromthymol blue yellow • Methyl orange red • p. H above 7 • Litmus blue • Phelphthalein pink • Bromthymol blue • Methyl orange yellow