Aim How to determine Empirical and Molecular Formula







- Slides: 12

Aim: How to determine Empirical and Molecular Formula • DO NOW: Copy the question. 1. Write the chemical formula for the structure to the right. 2. Re-write the formula but simplify the subscripts to have be in the smallest whole number ratio

Based on the do now, define: • Molecular formula- • Empirical formula- How to determine empirical and molecular formula

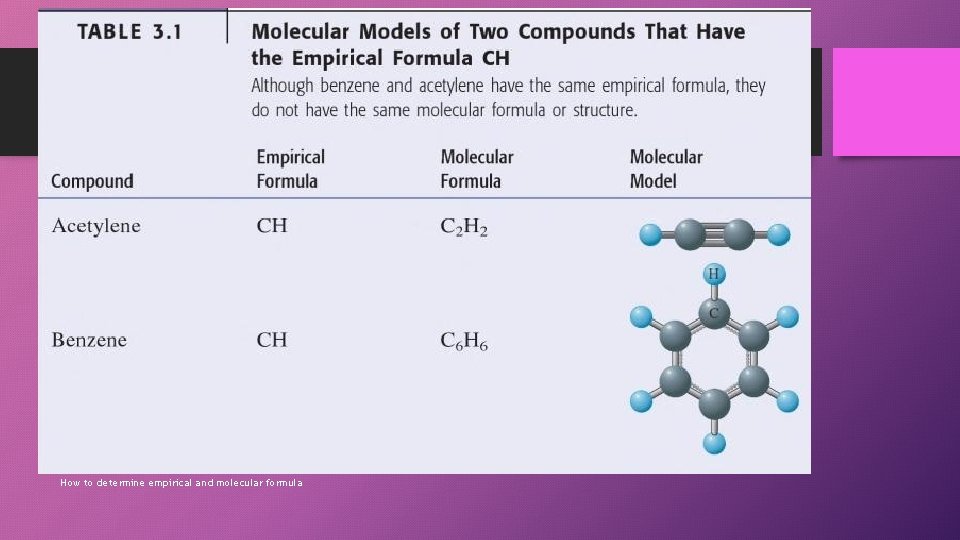
Empirical Formula vs. Molecular Formula • Empirical Formula tells us what elements are present in the compound in the simplest whole number ratio of elements. • Molecular formula tells which elements are present in the compound and the actual number of atoms each element. How to determine empirical and molecular formula

How to determine empirical and molecular formula

Determining empirical formula from molecular formula • Divide all the subscripts by their greatest common factor. How to determine empirical and molecular formula

Determining Empirical Formula from Molecular Formula 1. Which of the following substances has an empirical formula that is different from that of the others? a. C 4 H 8 O 4 b. C 3 H 6 O 2 c. C 2 H 4 O 2 d. C 6 H 12 O 6 How to determine empirical and molecular formula CH 2 O C 3 H 6 O 2 CH 2 O

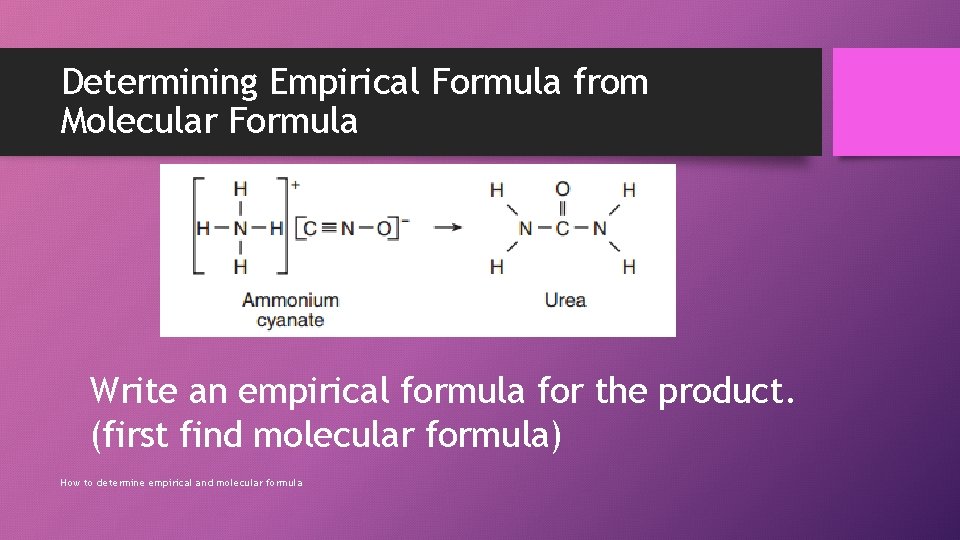
Determining Empirical Formula from Molecular Formula Write an empirical formula for the product. (first find molecular formula) How to determine empirical and molecular formula

Try Now In Pairs!! • Do the front page of the worksheet in pairs. One person does 1 -5, and the other one does 6 -10. After 3 min, you will be given a minute each to share your answers. • Hint for number 10, put your like elements together and then simplify. How to determine empirical and molecular formula

Determining Molecular Formula from Empirical Formula • The molecular formula is a multiple of empirical formula. • The molecular formula may be determined by dividing the molar mass of the compound by the empirical molar mass. When you divide you find which multiple it is of the empirical formula. How to determine empirical and molecular formula

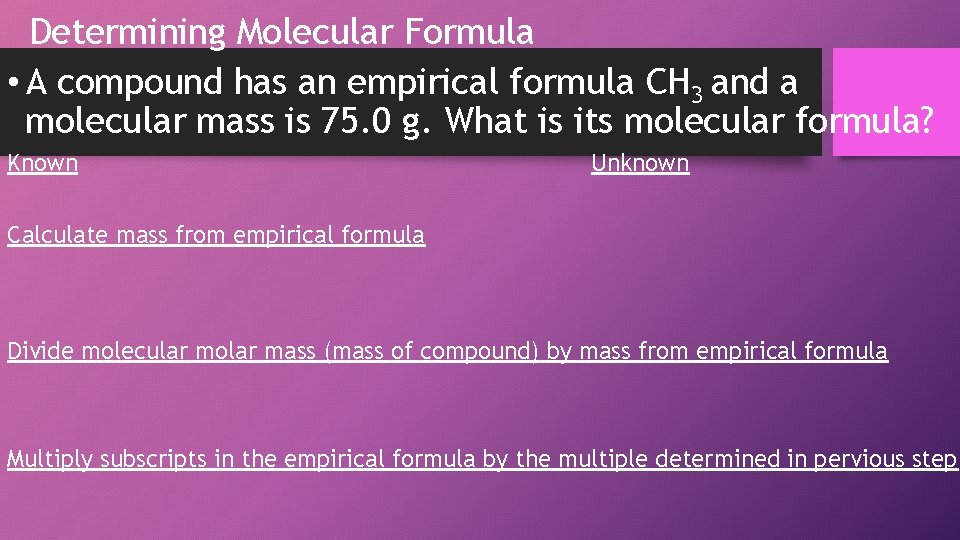
Determining Molecular Formula • A compound has an empirical formula CH 3 and a molecular mass is 75. 0 g. What is its molecular formula? Known Unknown Calculate mass from empirical formula Divide molecular molar mass (mass of compound) by mass from empirical formula Multiply subscripts in the empirical formula by the multiple determined in pervious step

Practice • What is the molecular formula of a compound that has an empirical formula of NO 2 and molecular mass of 92. 0 g? How to determine empirical and molecular formula

Practice • Determine the molecular formula for a compound that has the empirical formula CH 2 O and a molar mass of 120. grams per mole. How to determine empirical and molecular formula