Aim How to describe the polarity of a
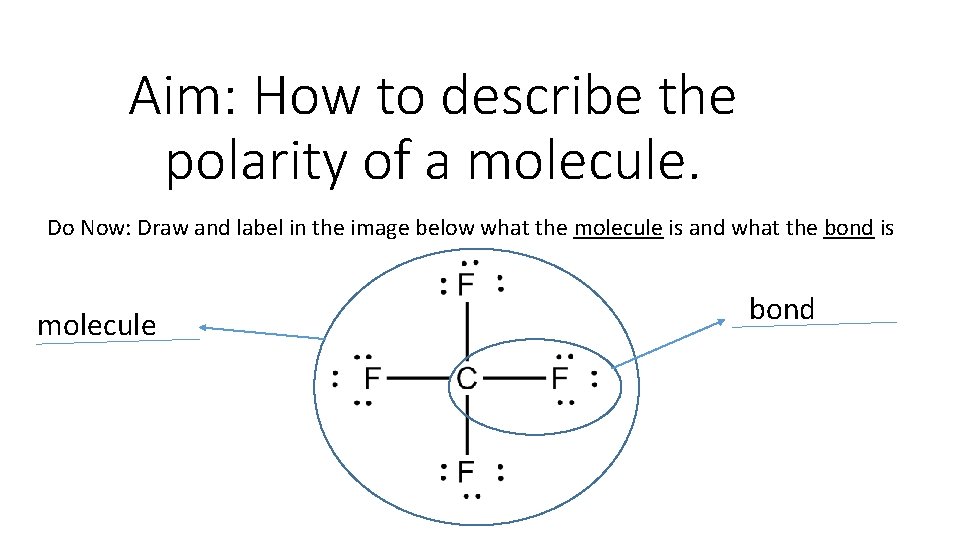
Aim: How to describe the polarity of a molecule. Do Now: Draw and label in the image below what the molecule is and what the bond is molecule bond
Definition of bond and molecule • The force that holds atoms together in collections known as molecules is referred to as a chemical bond. • A chemical bond is the attraction between two atoms.
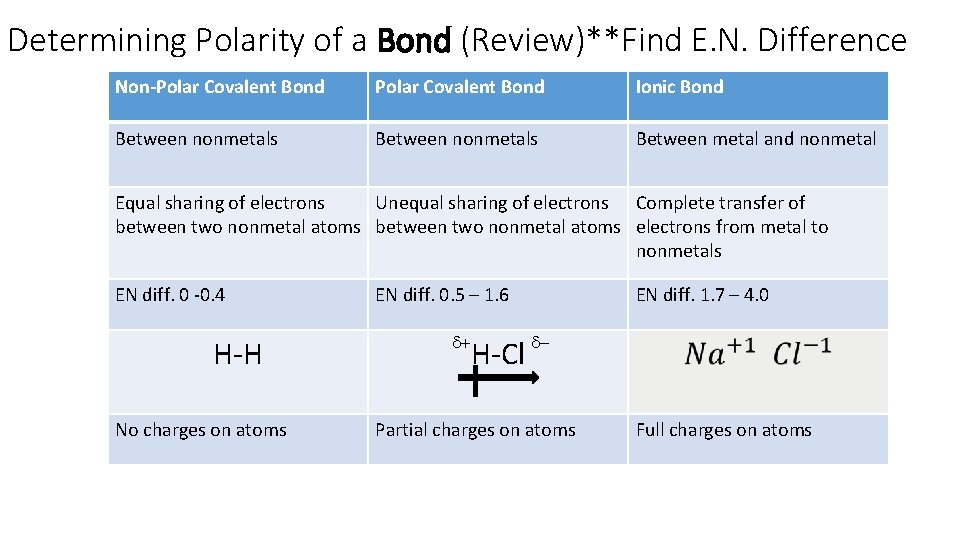
Determining Polarity of a Bond (Review)**Find E. N. Difference Non-Polar Covalent Bond Ionic Bond Between nonmetals Between metal and nonmetal Equal sharing of electrons Unequal sharing of electrons Complete transfer of between two nonmetal atoms electrons from metal to nonmetals EN diff. 0 -0. 4 H-H No charges on atoms EN diff. 0. 5 – 1. 6 d+ EN diff. 1. 7 – 4. 0 H-Cl d- Partial charges on atoms Full charges on atoms

Symmetric vs Asymmetric molecules • Think about what the terms symmetric and asymmetric mean. Next, determine if the following molecules are symmetric or asymmetric. Asym Symmetric

Polar Molecules • Molecules with a positive and a negative end • Requires two things to be true ¬The molecule must contain polar bonds This can be determined from differences in electronegativity. Asymmetric molecule.
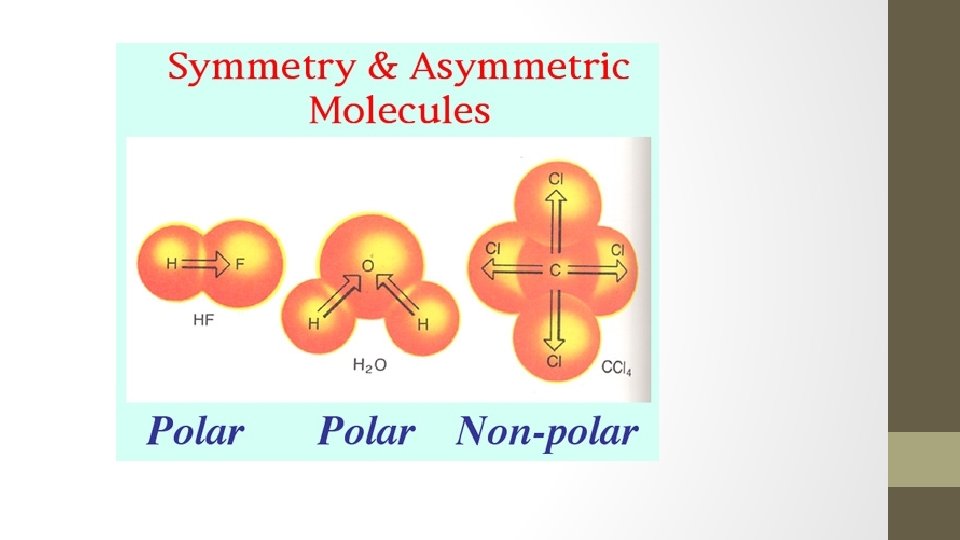
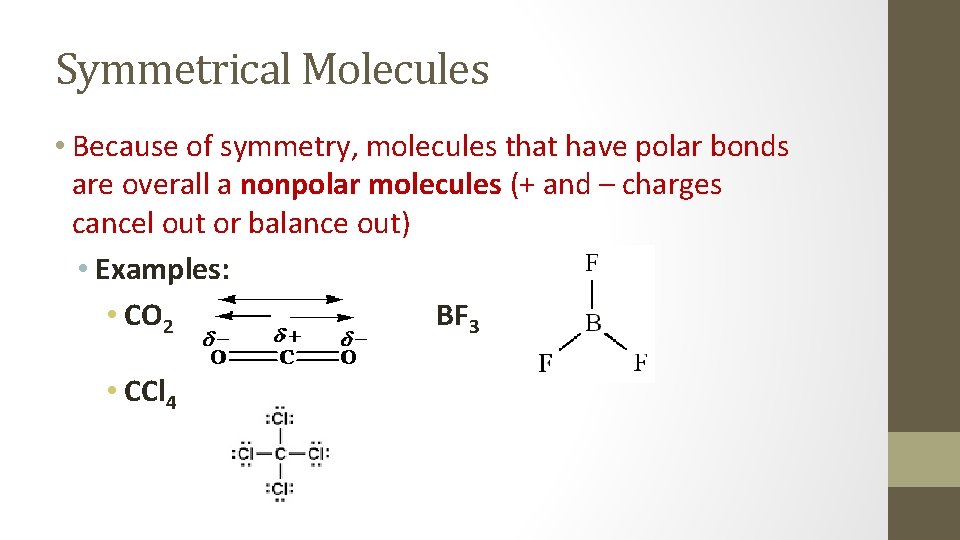
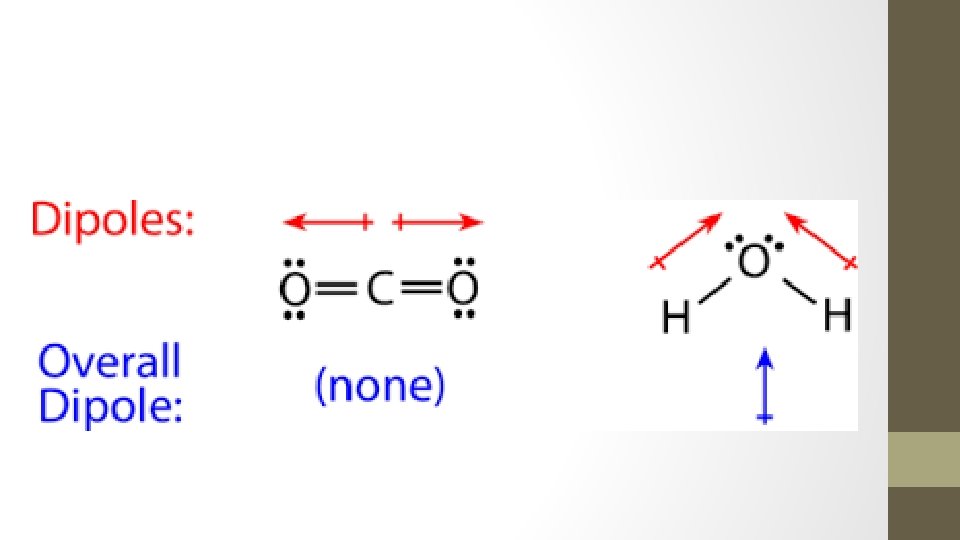
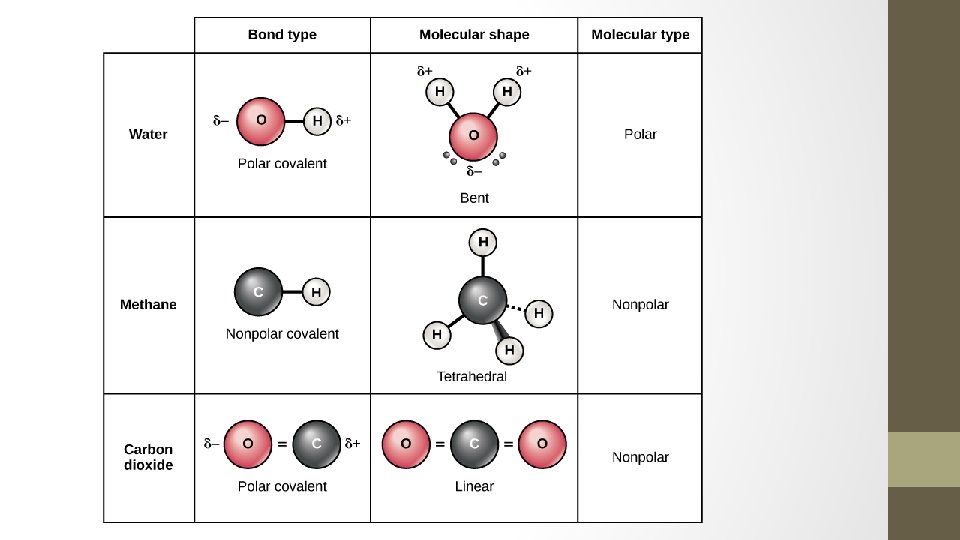
Symmetrical Molecules • Because of symmetry, molecules that have polar bonds are overall a nonpolar molecules (+ and – charges cancel out or balance out) • Examples: • CO 2 BF 3 • CCl 4
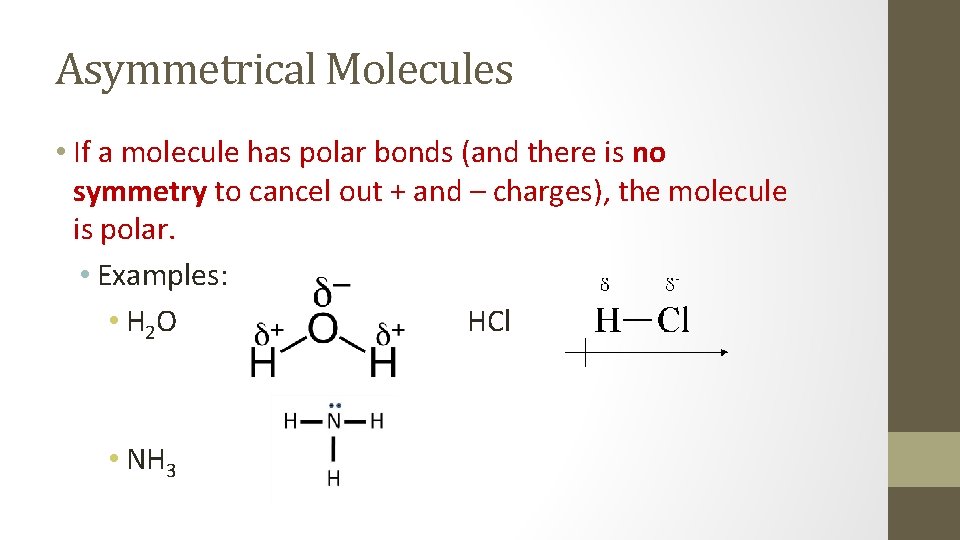
Asymmetrical Molecules • If a molecule has polar bonds (and there is no symmetry to cancel out + and – charges), the molecule is polar. • Examples: • H 2 O HCl • NH 3
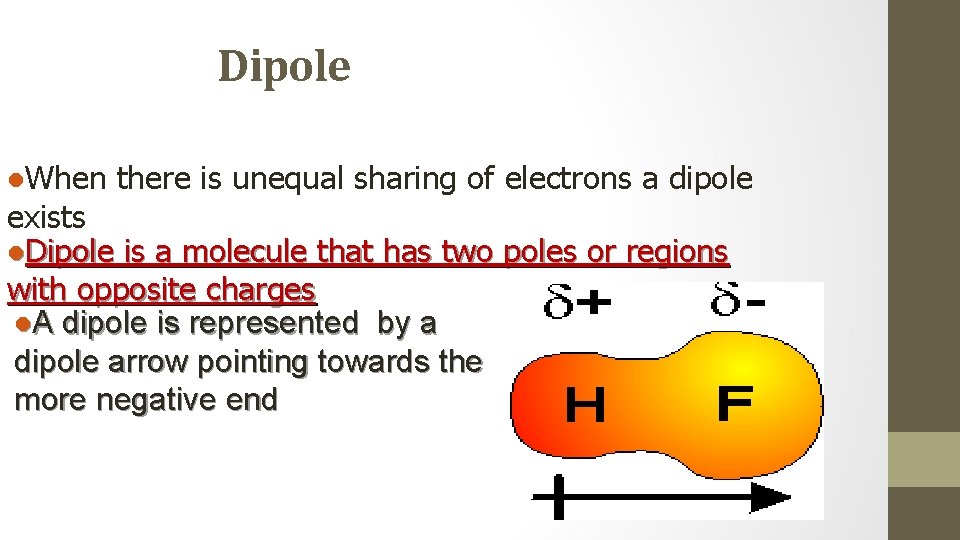
Dipole l. When there is unequal sharing of electrons a dipole exists l. Dipole is a molecule that has two poles or regions with opposite charges l. A dipole is represented by a dipole arrow pointing towards the more negative end
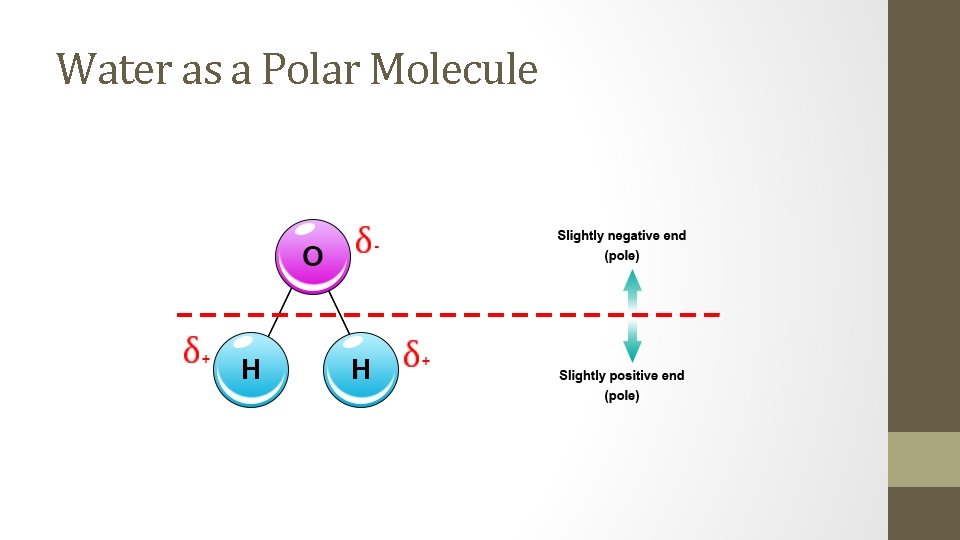
Water as a Polar Molecule
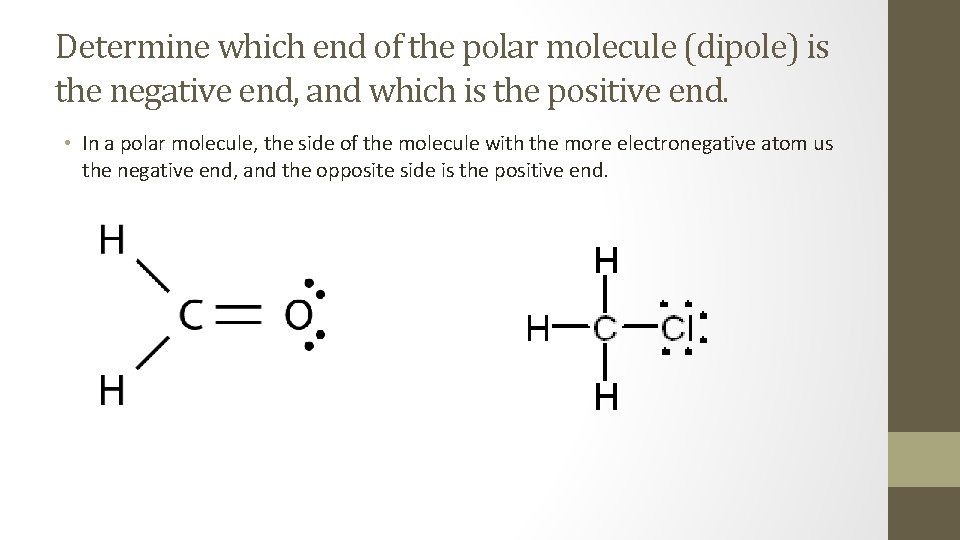
Determine which end of the polar molecule (dipole) is the negative end, and which is the positive end. • In a polar molecule, the side of the molecule with the more electronegative atom us the negative end, and the opposite side is the positive end.

Try in Pairs! • Read and highlight key terms on the “Recognizing Polar Molecules” worksheet. • Now, work on the worksheet. One person in in the pair works on the left half of the worksheet, while the other person works on the right half. When completed call me over so I can check your work. When both of you are done, discuss your answers.

Answer in Notebook • How do you determine if a bond is nonpolar, or ionic? • How do you determine if a molecule is polar or nonpolar? • Why would a molecule with polar bonds not necessarily be a polar molecule? Explain in terms of symmetry.

Summary • In a polar bond, one atom is more electronegative than the other. • In a nonpolar bond, both atoms have similar electronegativities. • An asymmetric molecule is a polar molecule. • A symmetric molecule is always a nonpolar molecule.
- Slides: 16