Aim How do we name ionic compounds Do





















- Slides: 32

Aim: How do we name ionic compounds? Do now: Write the chemical formulas for the following word equations: calcium and selenium Beryllium and sulfur Announcement: Quiz today Home work: Pg. 25 do numbers 16 -19

Ban DHMO? • DHMO is dihydrogen monoxide. • Evaluate the following link and decide if this potentially dangerous chemical should be prohibited. • www. dhmo. org

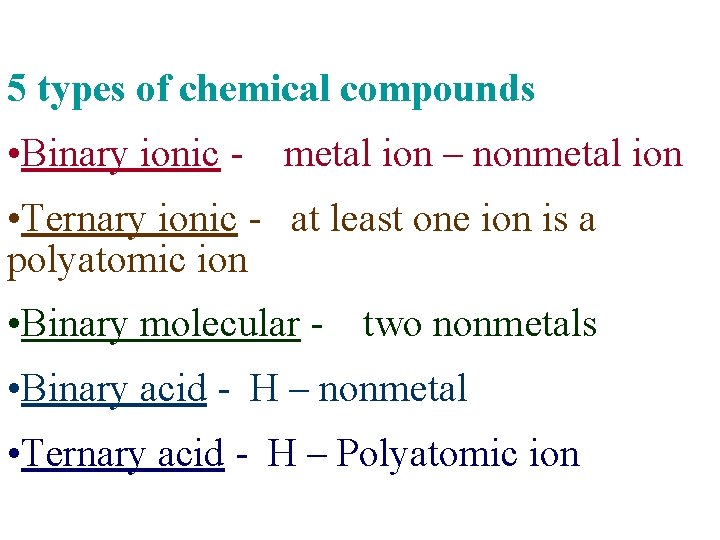
5 types of chemical compounds • Binary ionic - metal ion – nonmetal ion • Ternary ionic - at least one ion is a polyatomic ion • Binary molecular - two nonmetals • Binary acid - H – nonmetal • Ternary acid - H – Polyatomic ion

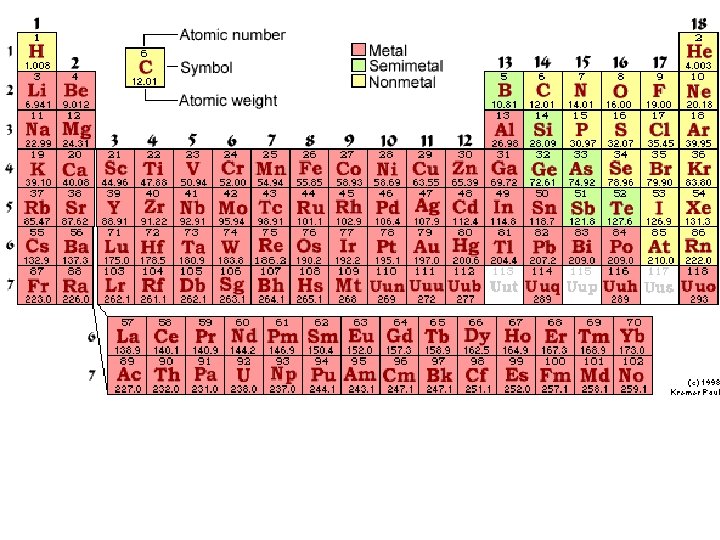
Tips to keep in mind when naming and writing formulas: Always keep your Periodic Table handy – You should have it in front of you ALWAYS when you are naming and writing formulas. Remember that metals (except Hydrogen) are found to the left of the stairstep on the Periodic Table. Nonmetals are found on the right side of the Periodic Table. Transition Metals are found from Group 3 to the stairstep (except aluminum which is a regular metal)


How do you name binary ionic compounds? (composed of two elements – a metal and a nonmetal) • Name the first ion (metal) • If the first ion is a transition element other than zinc, cadmium, or silver, you must use a Roman Numeral with the name – we’ll discuss this later. • Name the second ion (non-metal) changing the suffix to –ide.

Examples Na. Cl Sodium Chloride HIJKLMNO? Name the metal ion WATER – “H” to “O” Ca. O Calcium Oxide Al 2 S 3 Aluminum Sulfide Mg. I 2 Magnesium Iodide Ba. Na 2 What is the name of this compound: You have to admit – that was funny! Name the nonmetal ion, changing the suffix to –ide. This is two of metals not a binary ionic Theshould name this is– Banana (JOKE –this haha) You recognize a problem with one compound

What about the transition metals and using roman numerals? How does that work? Let’s see. Fe. O Iron II Oxide Notice – metal and nonmetal. Name the first ion. Since the first ion is a transition element, you must use a Roman Numeral to represent the charge. How do you know the charge? Deductive reasoning. Isn’t this easy and FUN!!! All compounds are neutral. Oxygen has a -2 charge (group 16) Therefore …. . Iron must have a +2 charge since there is one iron and one oxygen. Iron gets a Roman Numeral II.

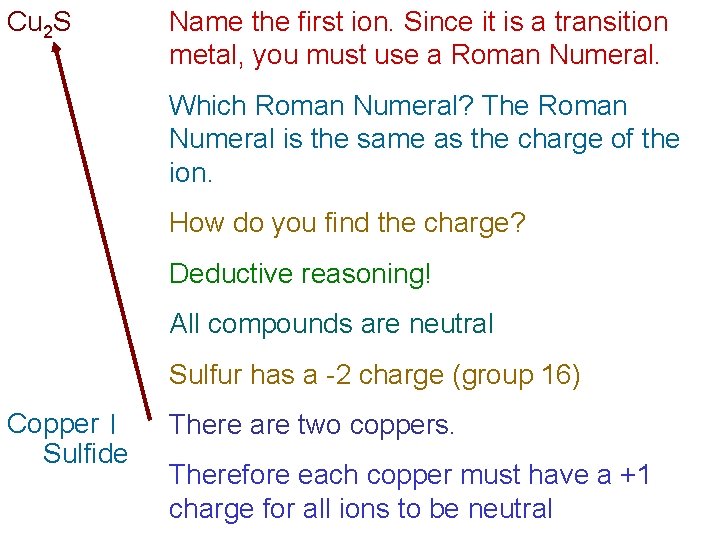
Cu 2 S Name the first ion. Since it is a transition metal, you must use a Roman Numeral. Which Roman Numeral? The Roman Numeral is the same as the charge of the ion. How do you find the charge? Deductive reasoning! All compounds are neutral Sulfur has a -2 charge (group 16) Copper I Sulfide There are two coppers. Therefore each copper must have a +1 charge for all ions to be neutral

Another Example: Mn. O 2 Name the first ion. Since it is a transition metal, you must use a Roman Numeral. Manganese IV oxide How do you determine the Roman Numeral? It is the same as the charge. What is the charge of Mn? All compounds are neutral. Oxygen (group 16) has a -2 charge. There are two oxygens and one Mn. Therefore Mn must have a +4 charge for this compound to be neutral.


Aim: How do we name polyatomic ions and covalent compounds? Do now: Name the following compound: Cu. O. Announcement: Homework - worksheet Thursday - Lab

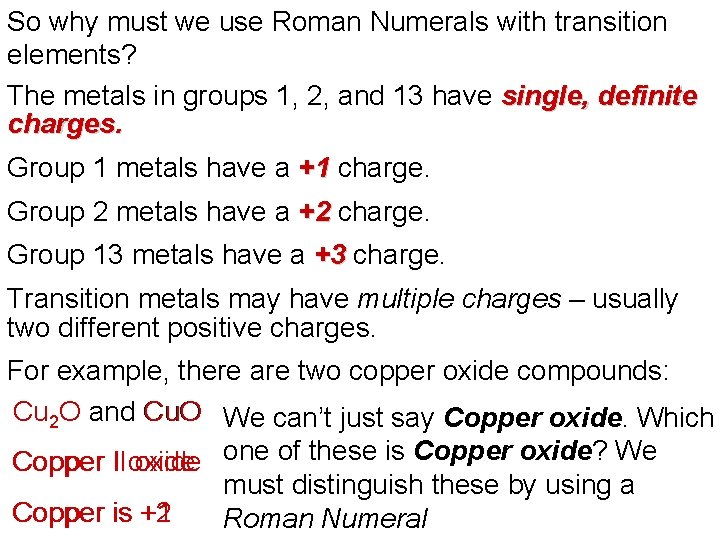
So why must we use Roman Numerals with transition elements? The metals in groups 1, 2, and 13 have single, definite charges. Group 1 metals have a +1 charge. Group 2 metals have a +2 charge. Group 13 metals have a +3 charge. Transition metals may have multiple charges – usually two different positive charges. For example, there are two copper oxide compounds: Cu 2 O and Cu. O We can’t just say Copper oxide. Which Copper II I oxide one of these is Copper oxide? We must distinguish these by using a Copper is +2 +1 Roman Numeral

There are three transition elements which do not require a Roman Numeral because they have single definite charges. These are Zinc – Zn+2 Cadmium – Cd+2 Silver – Ag+1 Ag 2 O Silver oxide Zn. Cl 2 Zinc chloride You need to remember the charges for these. No Name the second ion Roman changing the suffix to –ide. Numerals Name the first ion. needed Name the second ion for these. Name the first ion. changing the suffix to –ide.

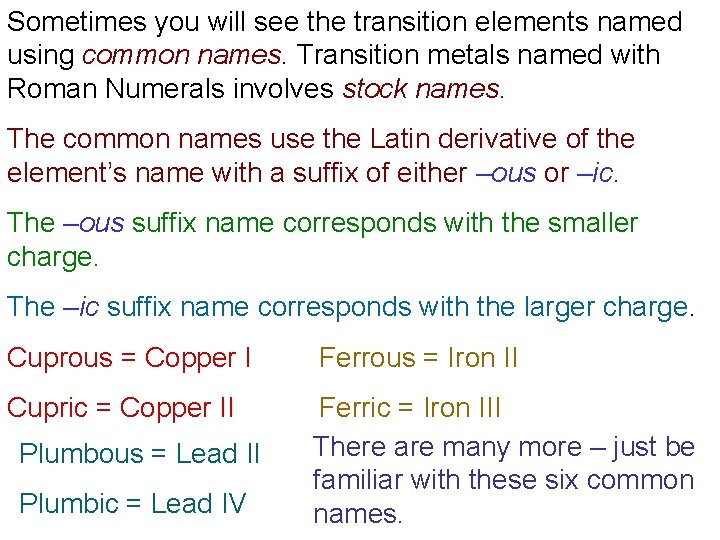
Sometimes you will see the transition elements named using common names. Transition metals named with Roman Numerals involves stock names. The common names use the Latin derivative of the element’s name with a suffix of either –ous or –ic. The –ous suffix name corresponds with the smaller charge. The –ic suffix name corresponds with the larger charge. Cuprous = Copper I Ferrous = Iron II Cupric = Copper II Ferric = Iron III There are many more – just be familiar with these six common names. Plumbous = Lead II Plumbic = Lead IV

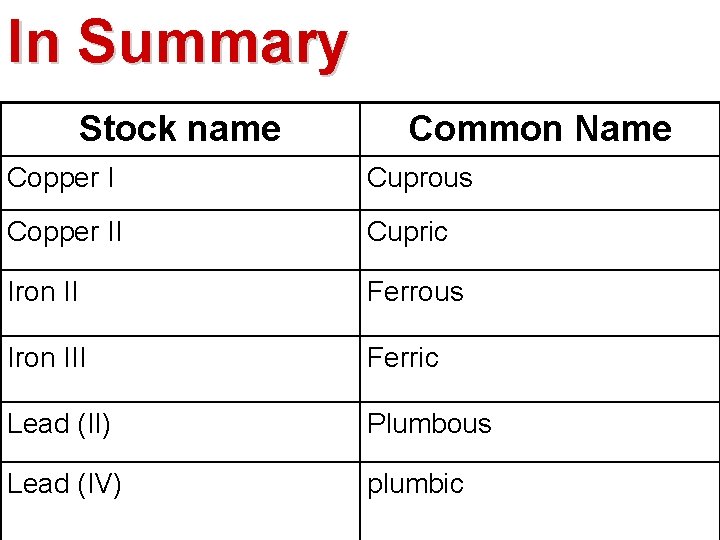
In Summary Stock name Common Name Copper I Cuprous Copper II Cupric Iron II Ferrous Iron III Ferric Lead (II) Plumbous Lead (IV) plumbic

How do you write formulas for binary ionic compounds given the name? Two simple steps: 1. Write the symbol and charge of each ion 2. Balancechloride the charges by providing Magnesium Write the symbol and charge of subscripts each ion. Mg+2 Cl-1 Balance the charges by supplying subscripts. Subscripts tell how many of each atom is present. -1 to balance You need a second Cl 2 the charges Mg. Cl

More examples: Iron III bromide Fe+3 Br -1 Fe. Br 3 Write the symbol and charge of each ion. The charge of the iron is provided by the Roman Numeral. Balance the charges by supplying subscripts. The subscripts tell how many of each ion is needed to balance the compound. You’ll need three bromine ions to balance the one iron.

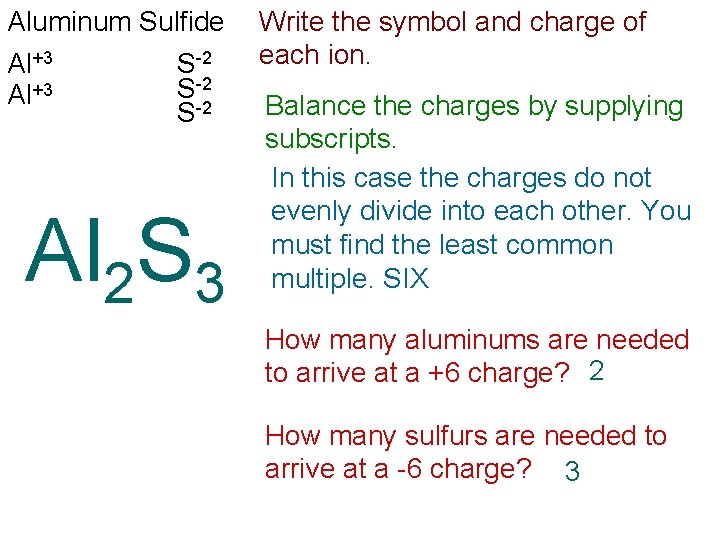
Aluminum Sulfide Al+3 S-2 Al 2 S 3 Write the symbol and charge of each ion. Balance the charges by supplying subscripts. In this case the charges do not evenly divide into each other. You must find the least common multiple. SIX How many aluminums are needed to arrive at a +6 charge? 2 How many sulfurs are needed to arrive at a -6 charge? 3

Second Category of compounds – Ternary Ionic Compounds. These compounds contain at least one polyatomic ion. What is a polyatomic ion? Let’s look at the name to try to understands. It is an ion – that means it has a charge. It is polyatomic – that means it is made of more than one atom. Simple as that!! Let’s look at some examples of polyatomic ions.

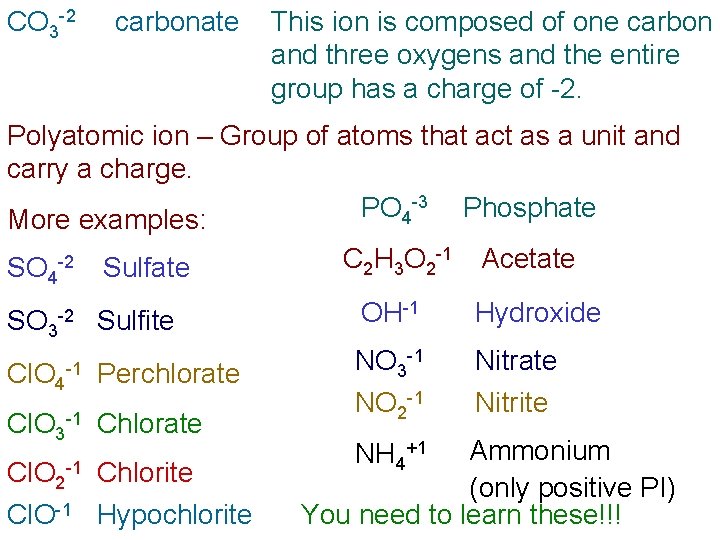
CO 3 -2 carbonate This ion is composed of one carbon and three oxygens and the entire group has a charge of -2. Polyatomic ion – Group of atoms that act as a unit and carry a charge. -3 PO Phosphate 4 More examples: SO 4 -2 SO 3 -2 Cl. O 4 -1 Sulfate C 2 H 3 O 2 -1 Acetate Sulfite OH-1 Hydroxide Perchlorate NO 3 -1 NO 2 -1 Nitrate Nitrite Cl. O 3 -1 Chlorate Cl. O 2 -1 Chlorite Cl. O-1 Hypochlorite Ammonium (only positive PI) You need to learn these!!! NH 4+1

How do you recognize Ternary Ionic Compounds? Composed of two ions in which at least one is a polyatomic ion. There is only one positive polyatomic ion (NH 4+1) Three possible types of Ternary Ionic Compounds: Polyatomic Ions • Ammonium + negative ion (nonmetal) • Metal (positive ion) + negative polyatomic ion • Ammonium + negative polyatomic ion

How do you name Ternary Ionic Compounds? EASY! PIECE OF CAKE! NO PROBLEM! Name the first ion. Name the second ion. Isn’t that simple? ? !! Examples: When you look at this compound you should recognize that this is NOT Na 2 CO 3 binary. There are THREE elements Sodium carbonate present. When you see this, immediately look for a polyatomic ion. Notice that you do Carbonate is present here. NOT change the suffix – just name Name the first ion. the polyatomic ion Name the second ion.

A few more examples: Fe(OH)3 Name the first ion. Iron III hydroxide Remember that iron requires a Roman Numeral since it is a transition Since there are element. What Roman Numeral 3 OH groups, should be used? each with a -1 The Roman Numeral comes from the charge, the charge of the ion. How do you find the charge of the iron? charge of the iron must be +3 You know two things: for the • All compounds are neutral. compound to • You know the charge of OH (-1) be neutral Name the polyatomic ion.

NH 4 Cl Name the first ion. Ammonium chloride Name the second ion. Notice that since the second ion is a nonmetal that, like binary ionic compounds, the suffix of the nonmetal changes to –ide. (NH 4)3 PO 4 Name the first ion. Ammonium phosphate Name the second ion. Looks like a monster, but it’s really a pussycat.

The Third Category of Compounds – Binary Molecular What are Binary Molecular Compounds? These compounds contain two elements (binary). The term “molecular” indicates that these elements are joined by a covalent bond. They must therefore be nonmetals. Bottom line – 2 elements – both nonmetals To name and write formulas for these, you must know some numerical prefixes.

Naming – you must use prefixes. 1 = mono 3 = tri 2 = di 5 = penta 4 = tetra 6 = hexa 7 = hepta 9 = nona 8 = octa 10 = deca Steps 1. The first nonmetal only gets a numeric prefix when there is more than one. No prefix if there is only one. 2. The second element always gets a numeric prefix and always has a suffix of ide

CO 2 Name the first element. Since there is only one, no prefix is Carbon dioxide needed. The second element always gets a prefix and a suffix of CO –ide. Carbon monoxide Name the first element. Since there are two present, N 2 O 4 the prefix “di” is needed. The second element always gets Dinitrogen tetraoxide a prefix and a suffix of –ide. H 2 O Dihydrogen monoxide Do you think it wise to BAN DHMO? CCl 4 Carbon tetrachloride

Al. Cl 3 What do you think about this one? ? ? Be careful. This is a metal and nonmetal. Always keep your Periodic Table in front of you for reference. You may have been tempted to say “aluminum trichloride”. This is INCORRECT! This is a binary IONIC compound. No prefixes are used. Simply aluminum chloride. Given the names of binary molecular compounds, how do you write the formulas? Very easy to do!!! The prefixes tell you how to write the formulas. DO NOT CONSIDER CHARGES. NONMETALS ARE ALL NEGATIVE SO TO USE CHARGES DOES NOT WORK!

Silicon dioxide Si. O 2 Silicon and oxygen are both nonmetals. The lack of a prefix on silicon means that there is only ONE silicon. The prefix “di” in front of oxide means that there are TWO oxygens. Diphosphorous pentachloride P 2 Cl 5 Phosphorous and chloride are both nonmetals. The prefix “di” means that there are TWO phosphorouses (Is that a word? ) The prefix “penta” before chlorine means that there are five chlorines.

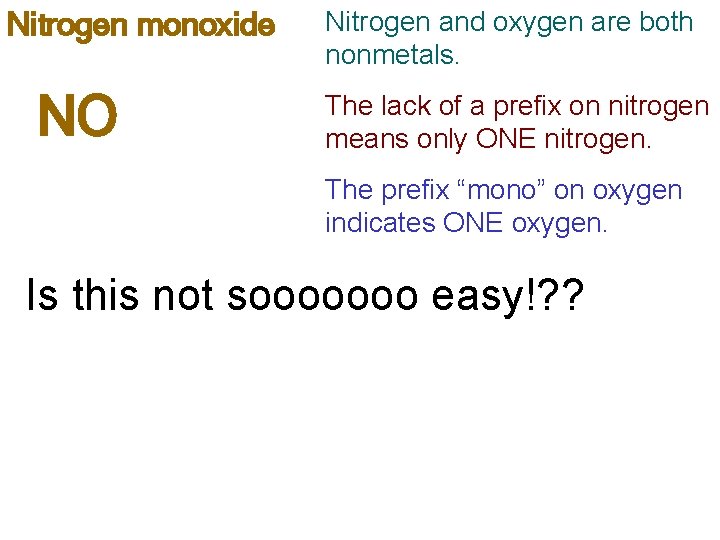
Nitrogen monoxide NO Nitrogen and oxygen are both nonmetals. The lack of a prefix on nitrogen means only ONE nitrogen. The prefix “mono” on oxygen indicates ONE oxygen. Is this not sooooooo easy!? ?

What are Binary Acids? Binary means two elements Acid means it contains hydrogen The second element is a nonmetal hydrogen – nonmetal Naming All binary acids follow the pattern as shown below: