Aim How do electrochemical cells use redox reactions






- Slides: 24

Aim: How do electrochemical cells use redox reactions to work? Do Now: Using Table J: 1. State which one will oxidize and which one will reduce in a voltaic cell. 2. State which one is the anode and which one is the cathode in a voltaic cell a. Calcium and iron c. Magnesium and Lead b. silver and nickel d. Copper and silver

Electrochemistry- involves a redox reaction and a flow of electrons

TWO TYPES of ELECTROCHEMICAL CELLS 1. Voltaic (similar to a battery)- spontaneous chemical reaction 2. Electrolytic (similar to alternator in cars) – nonspontaneous or forced chemical reaction

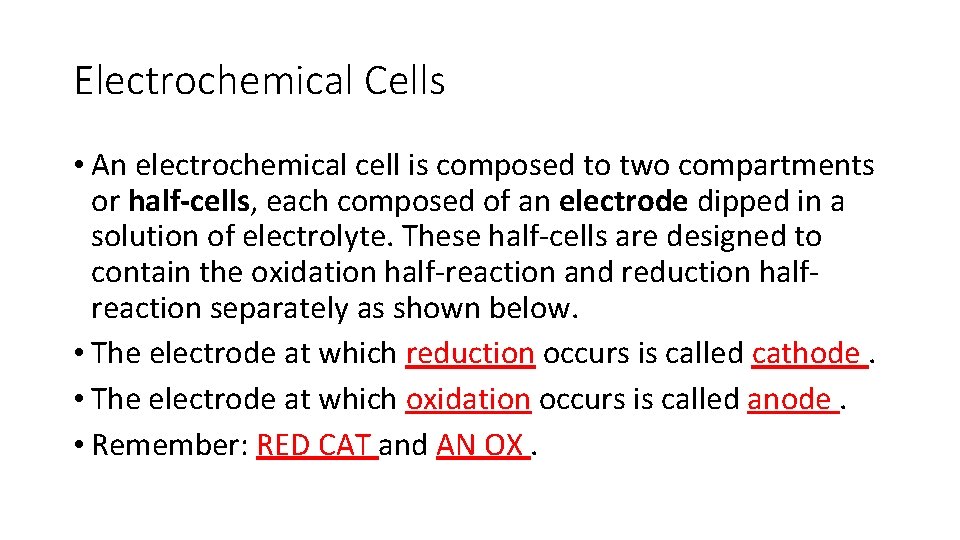
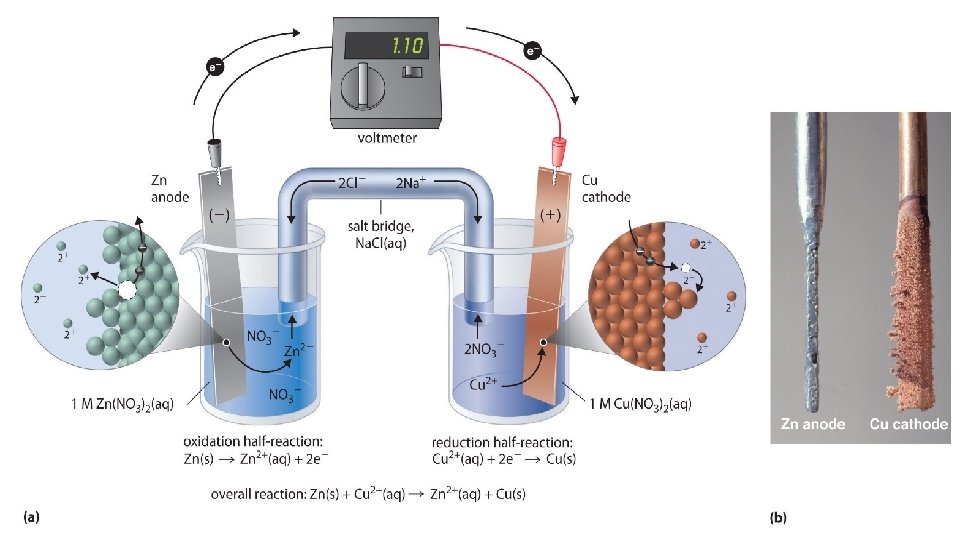
Electrochemical Cells • An electrochemical cell is composed to two compartments or half-cells, each composed of an electrode dipped in a solution of electrolyte. These half-cells are designed to contain the oxidation half-reaction and reduction halfreaction separately as shown below. • The electrode at which reduction occurs is called cathode. • The electrode at which oxidation occurs is called anode. • Remember: RED CAT and AN OX.

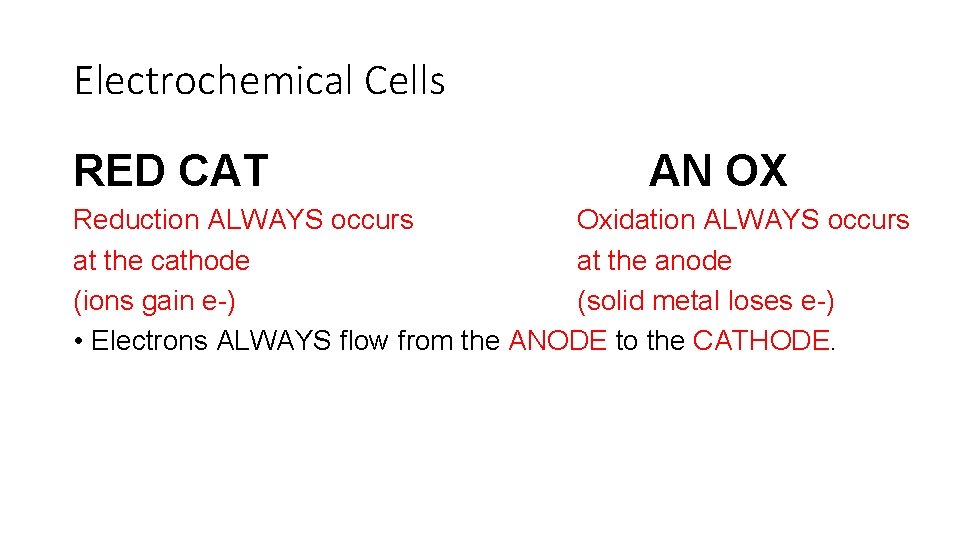
Electrochemical Cells RED CAT AN OX Reduction ALWAYS occurs Oxidation ALWAYS occurs at the cathode at the anode (ions gain e-) (solid metal loses e-) • Electrons ALWAYS flow from the ANODE to the CATHODE.

Voltaic Cell –Spontaneous Reactions • An electrochemical cell in which spontaneous chemical reaction produces a flow of electrons. In other words, chemical energy is converted to electrical energy/electricity. • The anode is designated as the negative end of the cell • The cathode is designated as the positive end of the cell

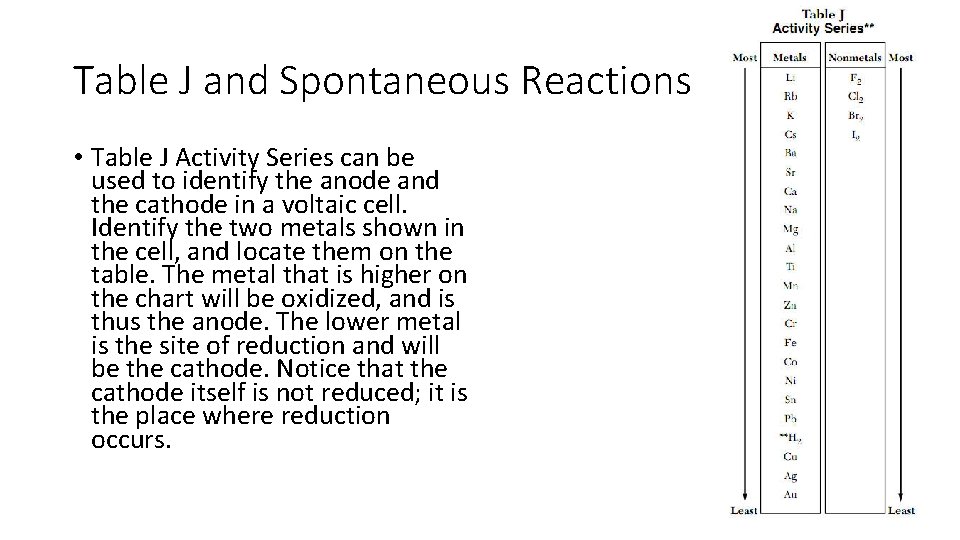
Table J and Spontaneous Reactions • Table J Activity Series can be used to identify the anode and the cathode in a voltaic cell. Identify the two metals shown in the cell, and locate them on the table. The metal that is higher on the chart will be oxidized, and is thus the anode. The lower metal is the site of reduction and will be the cathode. Notice that the cathode itself is not reduced; it is the place where reduction occurs.

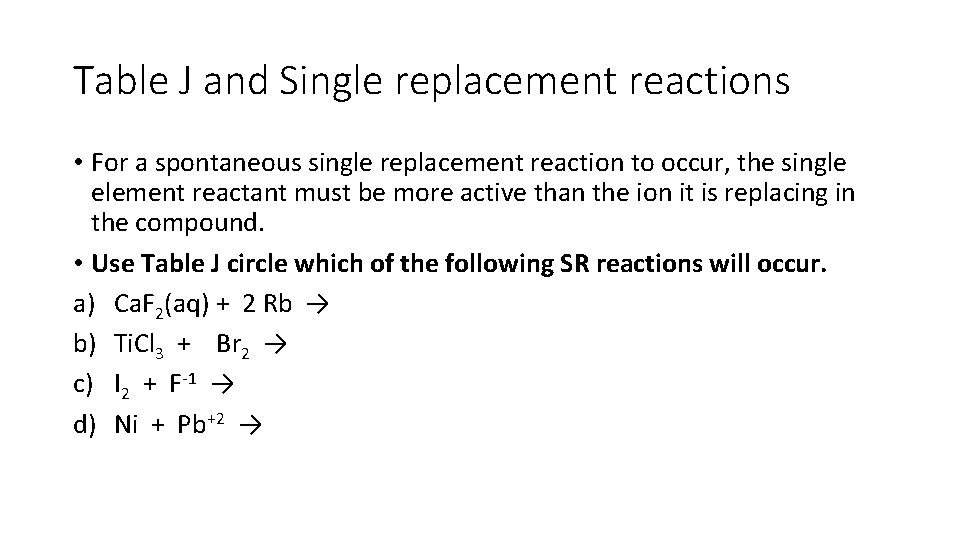
Table J and Single replacement reactions • For a spontaneous single replacement reaction to occur, the single element reactant must be more active than the ion it is replacing in the compound. • Use Table J circle which of the following SR reactions will occur. a) Ca. F 2(aq) + 2 Rb → b) Ti. Cl 3 + Br 2 → c) I 2 + F-1 → d) Ni + Pb+2 →


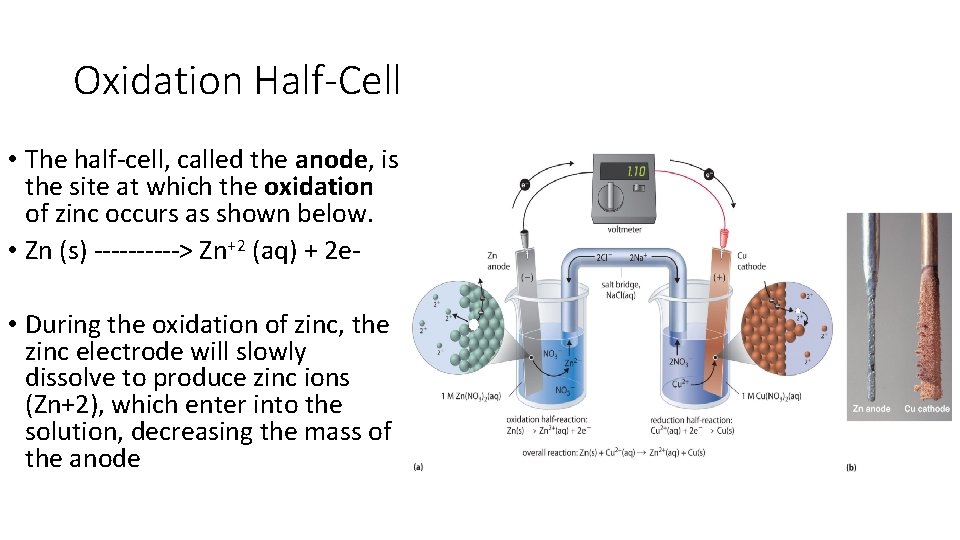
Oxidation Half-Cell • The half-cell, called the anode, is the site at which the oxidation of zinc occurs as shown below. • Zn (s) -----> Zn+2 (aq) + 2 e • During the oxidation of zinc, the zinc electrode will slowly dissolve to produce zinc ions (Zn+2), which enter into the solution, decreasing the mass of the anode

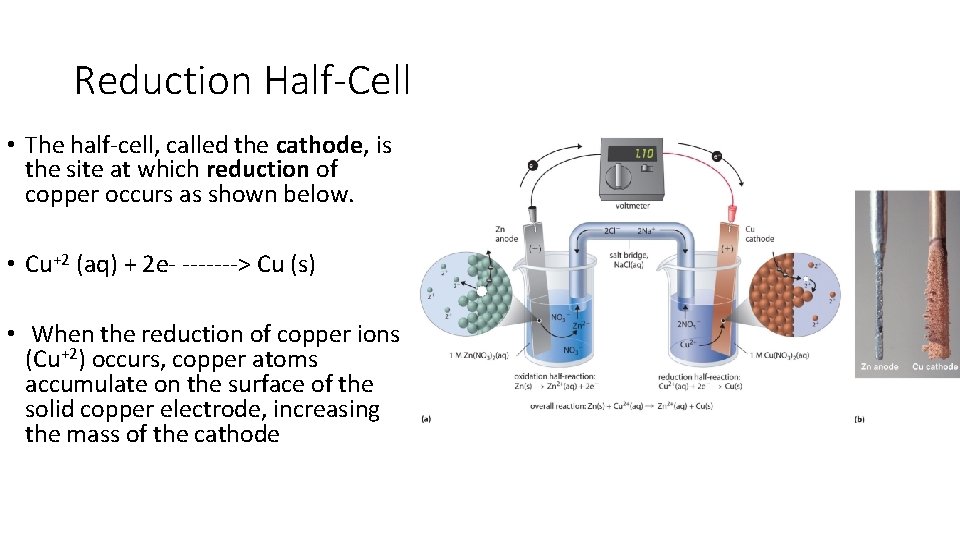
Reduction Half-Cell • The half-cell, called the cathode, is the site at which reduction of copper occurs as shown below. • Cu+2 (aq) + 2 e- -------> Cu (s) • When the reduction of copper ions (Cu+2) occurs, copper atoms accumulate on the surface of the solid copper electrode, increasing the mass of the cathode

Salt Bridge or Porous Membrane • The reaction in each half-cell does not occur unless the two half cells are connected to each other. • It is an inverted U-tube containing an electrolyte e. g KCl, KNO 3 etc it act as bridge by connecting two half cells Helps in: • To completing the electric circuit • To prevent mixing of solution of two half cell. • To help maintain electric neutrality

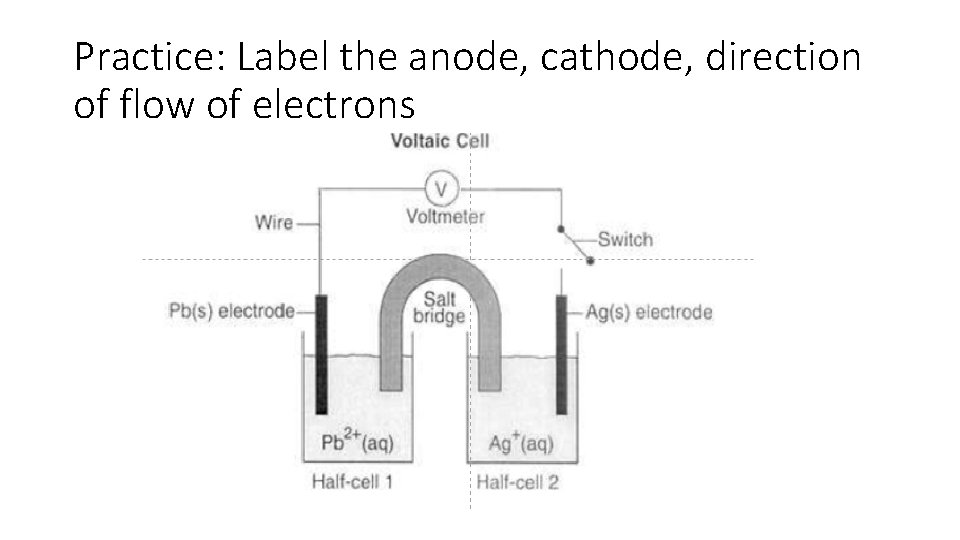
Practice: Label the anode, cathode, direction of flow of electrons

1. Use Table J to predict the direction that electrons will spontaneously flow. Draw arrows to indicate the direction on the wire. 2. Based on your answer above, which would be the negative electrode and which would be the positive electrode? _ the anode is the negative end and the cathode is the positive end 3. Explain your answer to #2. electrons move from the negative end to the positive end; therefore, the anode is the negative end and the cathode is the positive end. 4. At which electrode or in which half-cell does reduction occur? Half cell 2 5. At which electrode or in which half-cell does oxidation occur? _Half cell 1 6. Which electrode is the cathode? ___Ag____ 7. Which electrode is the anode? _____Pb______

Circle the correct answer • A (sponataneous/nonspontaneous) reaction occurs in the voltaic cell. • In a voltaic cell the anode is the cite of (oxidation/reduction), and is the (positive/negative) • In a voltaic cell the cathode is the cite of (oxidation/reduction), and is the (positive/negative) • The salt bridge allows the movement of (ions/electrons) between the two half cells. • The wire allows for the flow of (ions/electrons) between the two half cells. • Electrons always flow from (andoe/cathode) to (anode/cathode)

Electrolytic Cell • Reaction cannot occur spontaneously, so electricity from an external source is used to force the reaction to occur. In other words, electrical energy is converted to chemical energy. (opposite of voltaic cell) • When electricity is used to force a chemical reaction to occur, the process is called electrolysis.

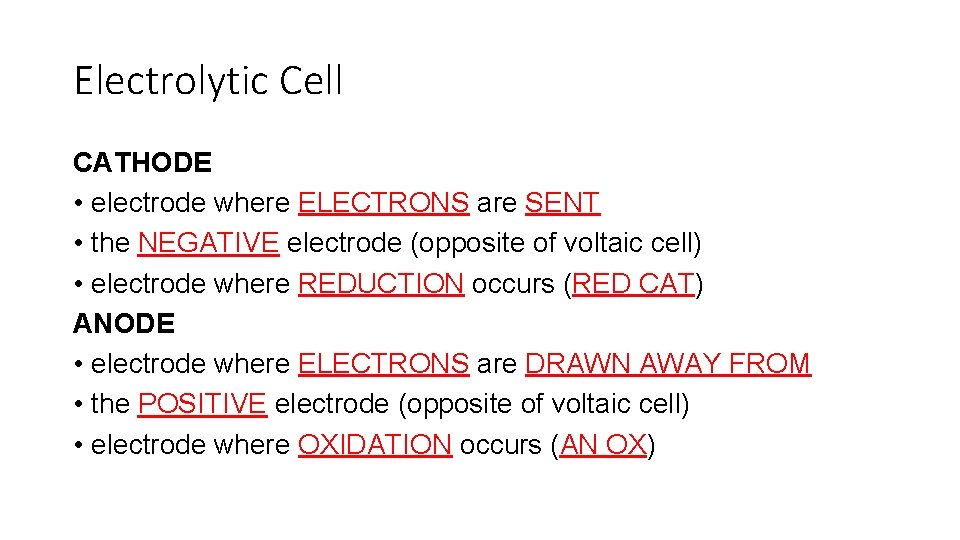
Electrolytic Cell CATHODE • electrode where ELECTRONS are SENT • the NEGATIVE electrode (opposite of voltaic cell) • electrode where REDUCTION occurs (RED CAT) ANODE • electrode where ELECTRONS are DRAWN AWAY FROM • the POSITIVE electrode (opposite of voltaic cell) • electrode where OXIDATION occurs (AN OX)

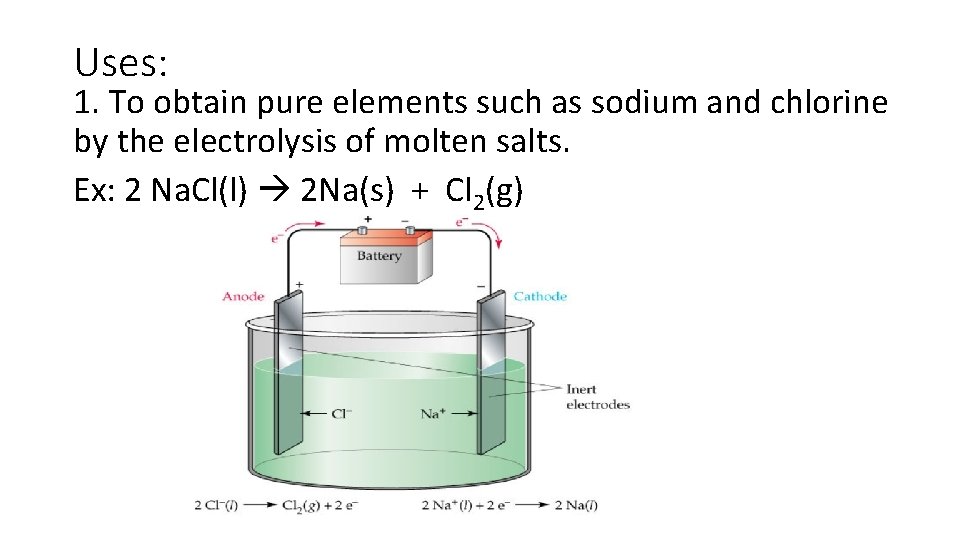
Uses: 1. To obtain pure elements such as sodium and chlorine by the electrolysis of molten salts. Ex: 2 Na. Cl(l) 2 Na(s) + Cl 2(g)

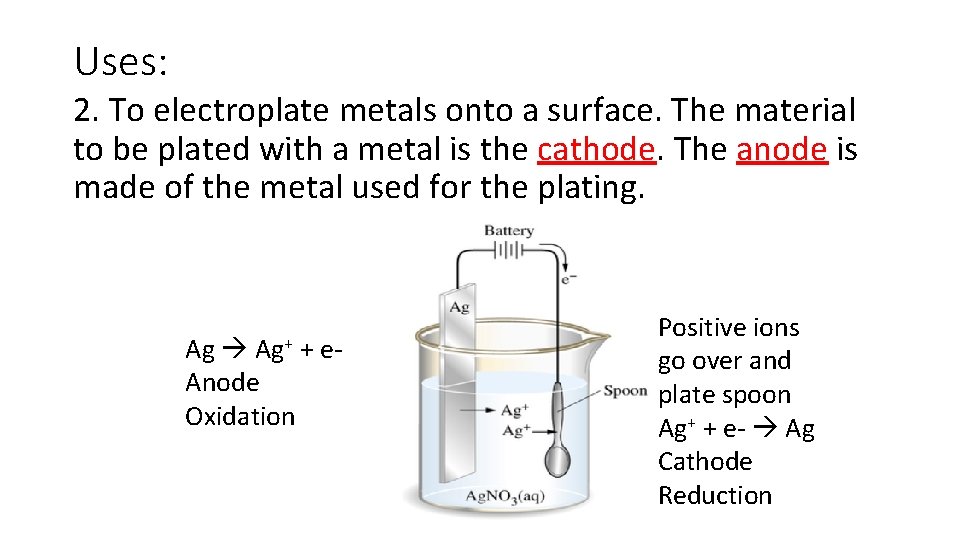
Uses: 2. To electroplate metals onto a surface. The material to be plated with a metal is the cathode. The anode is made of the metal used for the plating. Ag+ + e. Anode Oxidation Positive ions go over and plate spoon Ag+ + e- Ag Cathode Reduction

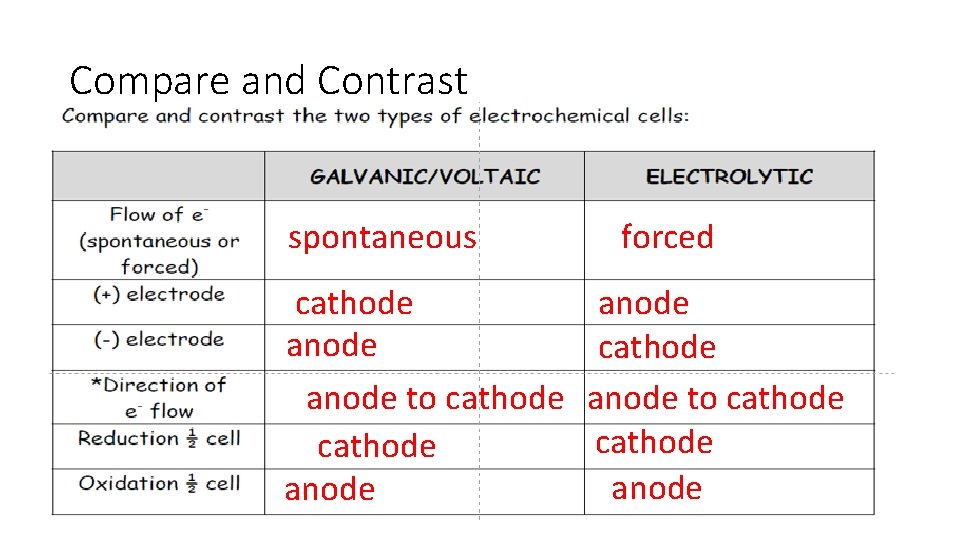
Compare and Contrast spontaneous cathode anode to cathode anode forced anode cathode anode to cathode anode

Practice 1. In an operating voltaic cell, reduction occurs a. at the anode b. at the cathode c. in the salt bridge d. in the wire 2. During the operation of a voltaic cell, the cell produces a. electrical energy spontaneously b. chemical energy spontaneously c. electrical energy nonspontaneously d. chemical energy nonspontaneously

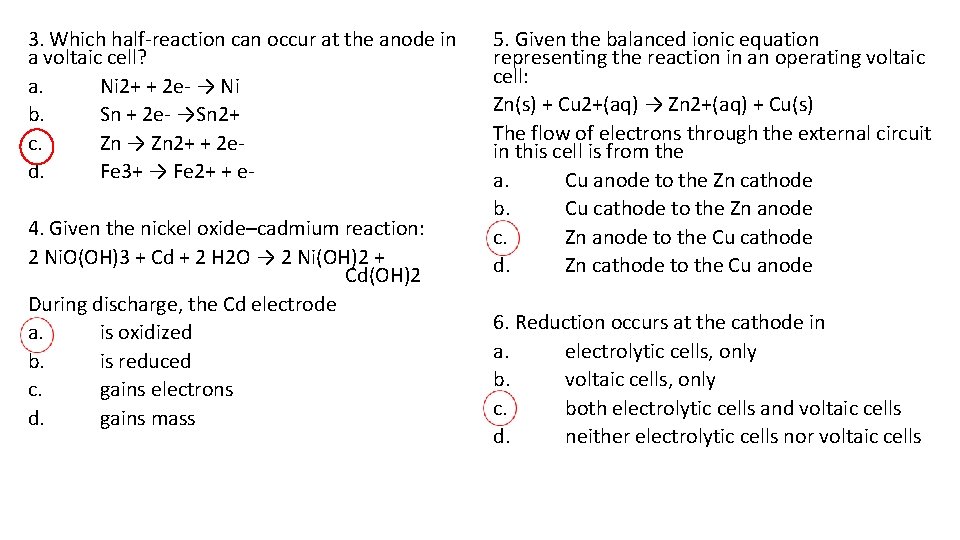
3. Which half-reaction can occur at the anode in a voltaic cell? a. Ni 2+ + 2 e- → Ni b. Sn + 2 e- →Sn 2+ c. Zn → Zn 2+ + 2 ed. Fe 3+ → Fe 2+ + e 4. Given the nickel oxide–cadmium reaction: 2 Ni. O(OH)3 + Cd + 2 H 2 O → 2 Ni(OH)2 + Cd(OH)2 During discharge, the Cd electrode a. is oxidized b. is reduced c. gains electrons d. gains mass 5. Given the balanced ionic equation representing the reaction in an operating voltaic cell: Zn(s) + Cu 2+(aq) → Zn 2+(aq) + Cu(s) The flow of electrons through the external circuit in this cell is from the a. Cu anode to the Zn cathode b. Cu cathode to the Zn anode c. Zn anode to the Cu cathode d. Zn cathode to the Cu anode 6. Reduction occurs at the cathode in a. electrolytic cells, only b. voltaic cells, only c. both electrolytic cells and voltaic cells d. neither electrolytic cells nor voltaic cells

7. Where does oxidation occur in an electrochemical cell? a. at the cathode in both an electrolytic cell and a voltaic cell b. at the cathode in an electrolytic cell and at the anode in a voltaic cell c. at the anode in both an electrolytic cell and a voltaic cell d. at the anode in an electrolytic cell and at the cathode in a voltaic cell 8. Which energy conversion must occur in an operating electrolytic cell? a. electrical energy to chemical energy b. electrical energy to nuclear energy c. chemical energy to electrical energy d. chemical energy to nuclear energy 9. Which statement describes one characteristic of an operating electrolytic cell? a. It produces electrical energy. b. It requires an external energy source. c. It uses radioactive nuclides. d. It undergoes a spontaneous redox reaction. 10. In an electrolytic cell, oxidation takes place at the a. anode, which is positive b. anode, which is negative c. cathode, which is positive d. cathode, which is negative

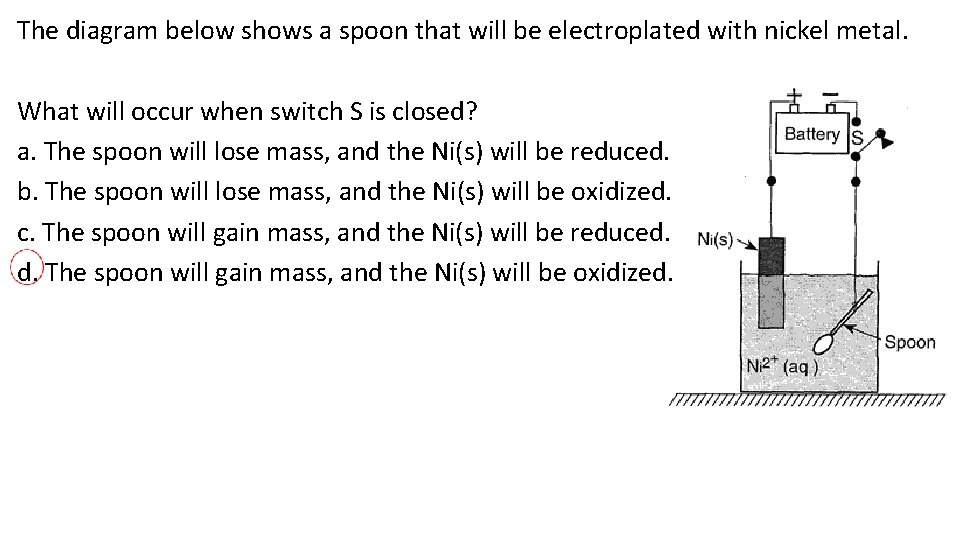
The diagram below shows a spoon that will be electroplated with nickel metal. What will occur when switch S is closed? a. The spoon will lose mass, and the Ni(s) will be reduced. b. The spoon will lose mass, and the Ni(s) will be oxidized. c. The spoon will gain mass, and the Ni(s) will be reduced. d. The spoon will gain mass, and the Ni(s) will be oxidized.