AIM DETERMINING IONIZATION ENERGY AND ELECTRONEGATIVITY OF ELEMENTS














- Slides: 18

AIM: DETERMINING IONIZATION ENERGY AND ELECTRONEGATIVITY OF ELEMENTS DO NOW: 1. LIST IN ORDER OF INCREASING ATOMIC RADII: MAGNESIUM, SILICON, BARIUM, BROMINE 2. DO NOW ON METALS, NONMETALS, AND METALLOIDS WORKSHEET

IONS • Positive and negative ions form when electrons are transferred between atoms. • Metals tend to lose electrons; nonmetals tend to gain those electrons

ANION • An ion with a negative charge • (N-3, O-2, Cl-1)

CATION • An ion with a positive charge • (Na+1, Ca+2)

IONIZATION ENERGY • The energy required to remove an electron from an atom. • The bigger the ionization energy value the harder to lose an electron (the stronger the atom is holding on to its electron)!

FIRST IONIZATION ENERGY • The first ionization energy is the energy required to remove the first electron from its atom. • First ionization energy tends to decrease from top to bottom within a group and increase from left to right across a period.

SECOND AND THIRD IONIZATION ENERGY • The second ionization energy is the energy required to remove an electron from an ion with a 1+ charge. • The third ionization energy is the energy required to remove an electron from an ion with a 2+ charge.

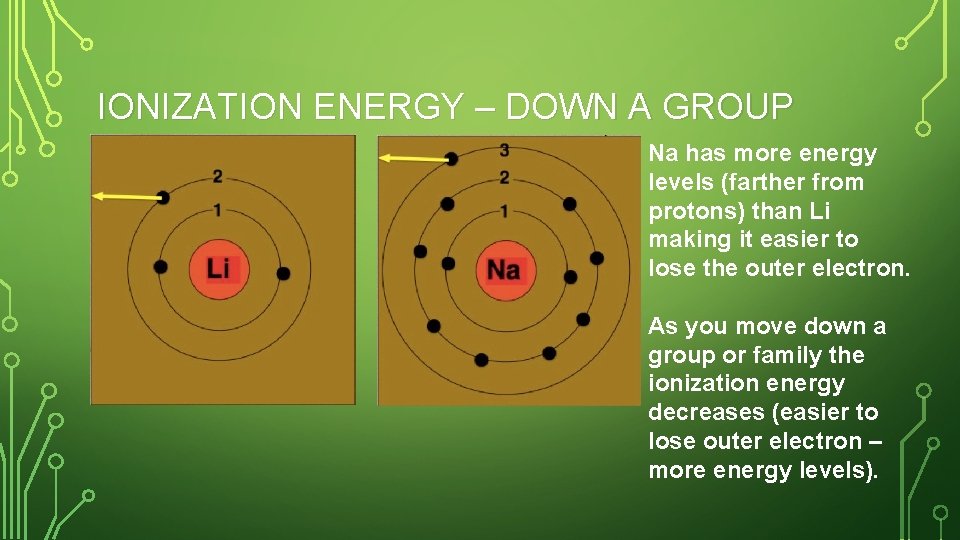
IONIZATION ENERGY – DOWN A GROUP Na has more energy levels (farther from protons) than Li making it easier to lose the outer electron. As you move down a group or family the ionization energy decreases (easier to lose outer electron – more energy levels).

IONIZATION ENERGY – ACROSS A PERIOD Both have the same number of energy levels, but Be has more protons pulling stronger on the outer most electron. As you move across a period, the ionization energy increases (herder to lose outer electron – more protons).


WHICH HAS LOWEST IONIZATION ENERGY? • Chlorine • Sodium • Magnesium • Argon

ELECTRONEGATIVITY • The ability of an atom to attract electrons when the atom is in a compound. • The ability of an atom to steal an electron from another atom (electron thieves).

ELECTRONEGATIVITY • In general, electronegativity values decrease from top to bottom within a group. For representative elements, the values tend to increase from left to right across a period.

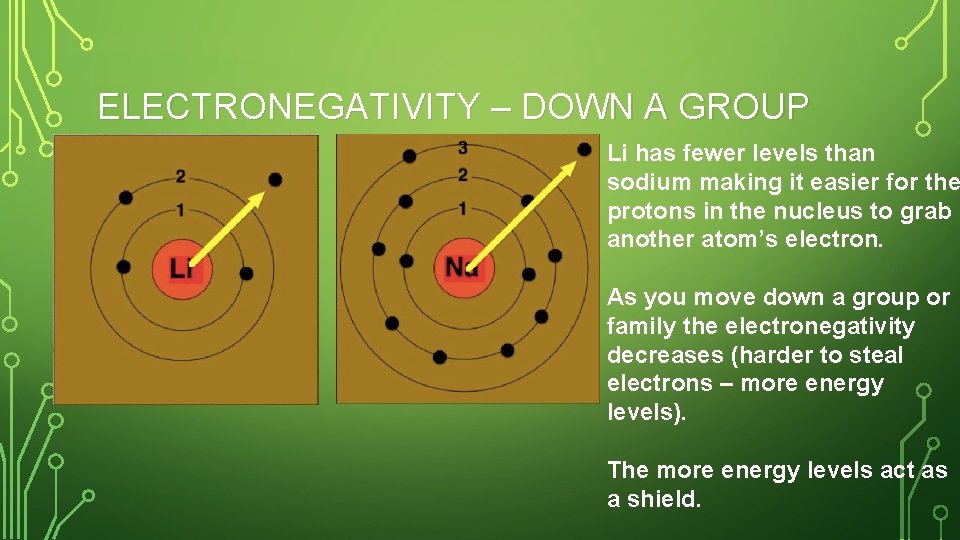
ELECTRONEGATIVITY – DOWN A GROUP Li has fewer levels than sodium making it easier for the protons in the nucleus to grab another atom’s electron. As you move down a group or family the electronegativity decreases (harder to steal electrons – more energy levels). The more energy levels act as a shield.

ELECTRONEGATIVITY – ACROSS A PERIOD Both have the same number of energy levels, but Be has more protons pulling stronger on another atom’s electron. As you move across a period, the electronegativity increases (easier to steal electrons – more protons). *Noble Gases do not have desire to steal electrons because of outer shell!*

WHICH HAS LARGEST ELECTRONEGATIVITY? • Chlorine • Sodium • Magnesium • Argon

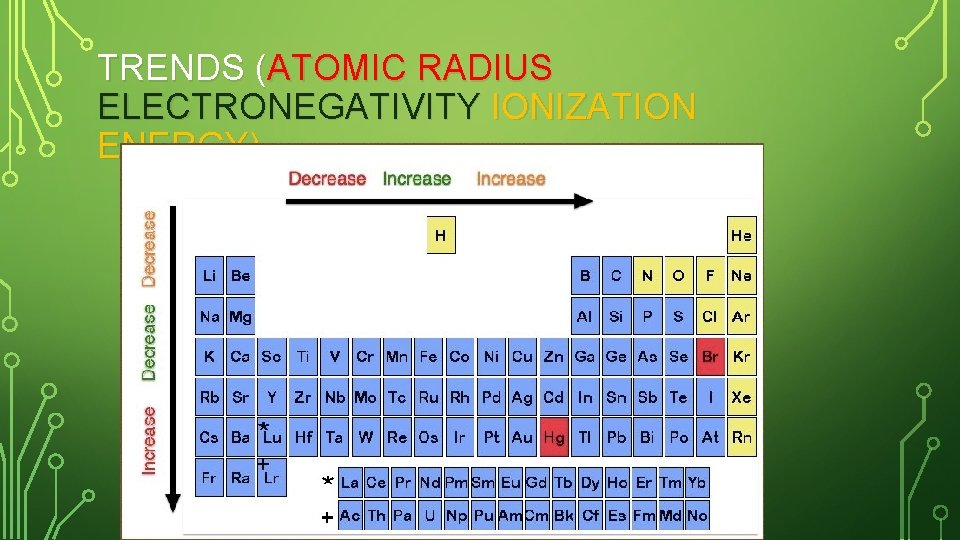
TRENDS (ATOMIC RADIUS ELECTRONEGATIVITY IONIZATION ENERGY)