Aim 6 How is a weak acid different
![II Calculating [H+] and p. H from Ka Problem: Find the p. H of II Calculating [H+] and p. H from Ka Problem: Find the p. H of](https://slidetodoc.com/presentation_image/05f150ea5cd3f5f432200d106f318dc3/image-4.jpg)
![% dissociation = [H+] x 100 [HA] 0 For the problem above, [H+] x % dissociation = [H+] x 100 [HA] 0 For the problem above, [H+] x](https://slidetodoc.com/presentation_image/05f150ea5cd3f5f432200d106f318dc3/image-6.jpg)

- Slides: 8

Aim # 6: How is a weak acid different from a strong acid? H. W. # 6 Study pp. 666 -675 (sec. 14. 5) Ans. ques. p. 704 # 65, 73, 75, 78, 79 Ka values are in appendix A 5. 1 (p. A 22) Do Now: What mass of HNO 3 is present in 250. 0 m. L of a nitric acid solution having a p. H of 5. 10?

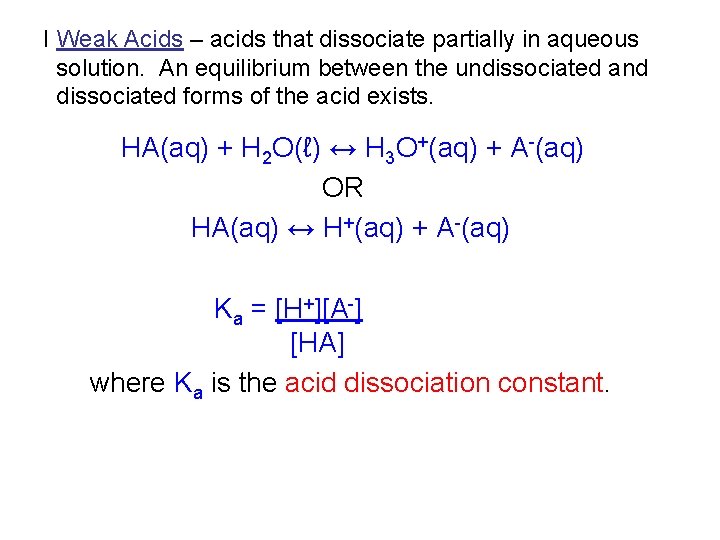
I Weak Acids – acids that dissociate partially in aqueous solution. An equilibrium between the undissociated and dissociated forms of the acid exists. HA(aq) + H 2 O(ℓ) ↔ H 3 O+(aq) + A-(aq) OR HA(aq) ↔ H+(aq) + A-(aq) Ka = [H+][A-] [HA] where Ka is the acid dissociation constant.

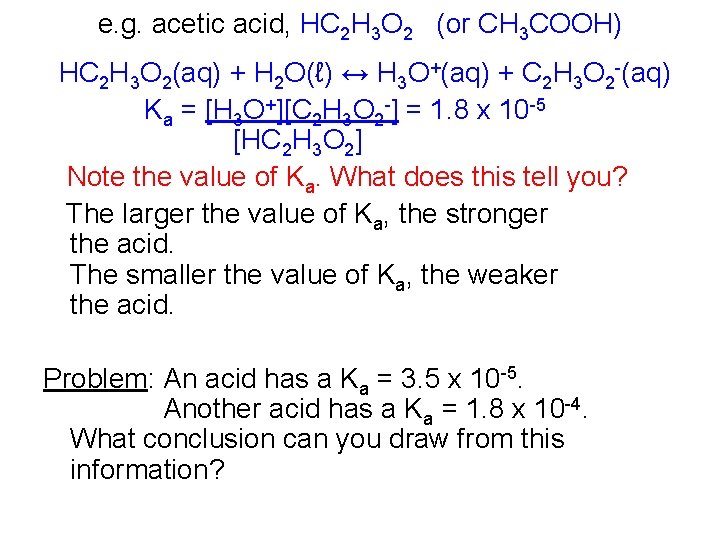
e. g. acetic acid, HC 2 H 3 O 2 (or CH 3 COOH) HC 2 H 3 O 2(aq) + H 2 O(ℓ) ↔ H 3 O+(aq) + C 2 H 3 O 2 -(aq) Ka = [H 3 O+][C 2 H 3 O 2 -] = 1. 8 x 10 -5 [HC 2 H 3 O 2] Note the value of Ka. What does this tell you? The larger the value of Ka, the stronger the acid. The smaller the value of Ka, the weaker the acid. Problem: An acid has a Ka = 3. 5 x 10 -5. Another acid has a Ka = 1. 8 x 10 -4. What conclusion can you draw from this information?
![II Calculating H and p H from Ka Problem Find the p H of II Calculating [H+] and p. H from Ka Problem: Find the p. H of](https://slidetodoc.com/presentation_image/05f150ea5cd3f5f432200d106f318dc3/image-4.jpg)
II Calculating [H+] and p. H from Ka Problem: Find the p. H of a 0. 200 M solution of formic acid, HCHO 2, whose Ka = 1. 76 x 10 -4. Ans: HCHO 2(aq) ↔ H+(aq) + CHO 2 -(aq) Ka = [H+][CH 2 O] = 1. 76 x 10 -4 [HCHO 2] HCHO 2 ↔ I 0. 200 M C -X E 0. 200 - X H+ 0 M +X X + CHO 2 - 0 M +X X

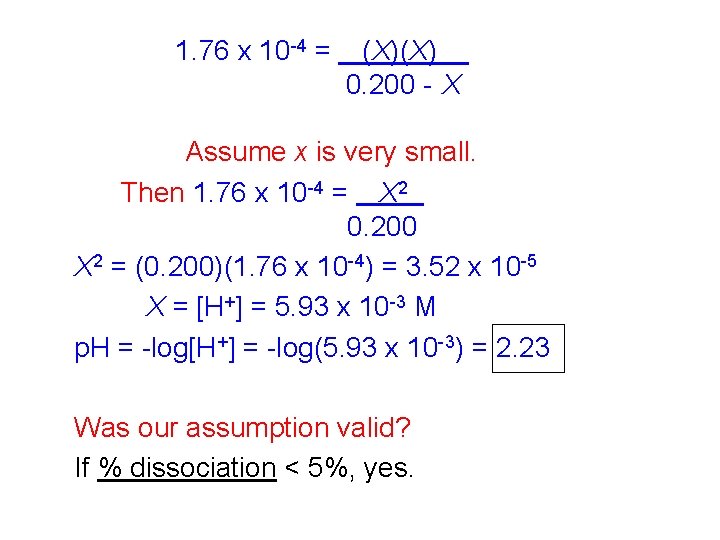
1. 76 x 10 -4 = (X)(X) 0. 200 - X Assume x is very small. Then 1. 76 x 10 -4 = X 2 0. 200 X 2 = (0. 200)(1. 76 x 10 -4) = 3. 52 x 10 -5 X = [H+] = 5. 93 x 10 -3 M p. H = -log[H+] = -log(5. 93 x 10 -3) = 2. 23 Was our assumption valid? If % dissociation < 5%, yes.
![dissociation H x 100 HA 0 For the problem above H x % dissociation = [H+] x 100 [HA] 0 For the problem above, [H+] x](https://slidetodoc.com/presentation_image/05f150ea5cd3f5f432200d106f318dc3/image-6.jpg)
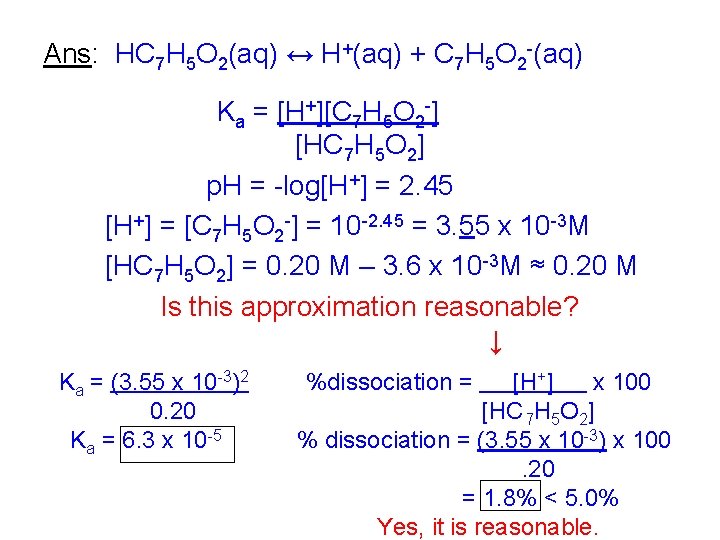
% dissociation = [H+] x 100 [HA] 0 For the problem above, [H+] x 100 = 5. 93 x 10 -3 x 100 = 2. 97% [HA]0 0. 200 < 5. 0% Yes, the assumption was valid. III Calculating Ka and % dissociation from p. H. Problem: The p. H of 0. 20 M benzoic acid is found to be 2. 45. Calculate Ka and the % of the acid which is dissociated.

Ans: HC 7 H 5 O 2(aq) ↔ H+(aq) + C 7 H 5 O 2 -(aq) Ka = [H+][C 7 H 5 O 2 -] [HC 7 H 5 O 2] p. H = -log[H+] = 2. 45 [H+] = [C 7 H 5 O 2 -] = 10 -2. 45 = 3. 55 x 10 -3 M [HC 7 H 5 O 2] = 0. 20 M – 3. 6 x 10 -3 M ≈ 0. 20 M Is this approximation reasonable? ↓ Ka = (3. 55 x 10 -3)2 0. 20 Ka = 6. 3 x 10 -5 %dissociation = [H+] x 100 [HC 7 H 5 O 2] % dissociation = (3. 55 x 10 -3) x 100. 20 = 1. 8% < 5. 0% Yes, it is reasonable.

Practice Problems: Zumdahl (8 th ed. ) p. 691 # 66, 69, 75, 78, 80