Aim 48 What are the advantages and disadvantages

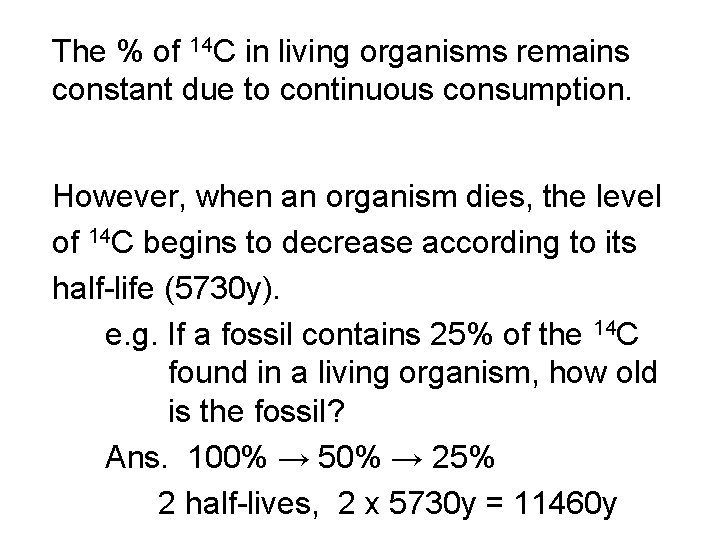

- Slides: 9

Aim # 48: What are the advantages and disadvantages of radioactive isotopes? H. W. # 48 Study pp. 621 -626 Ans. ques. p. 630 # 27 -32, 34, 53, 54, 56

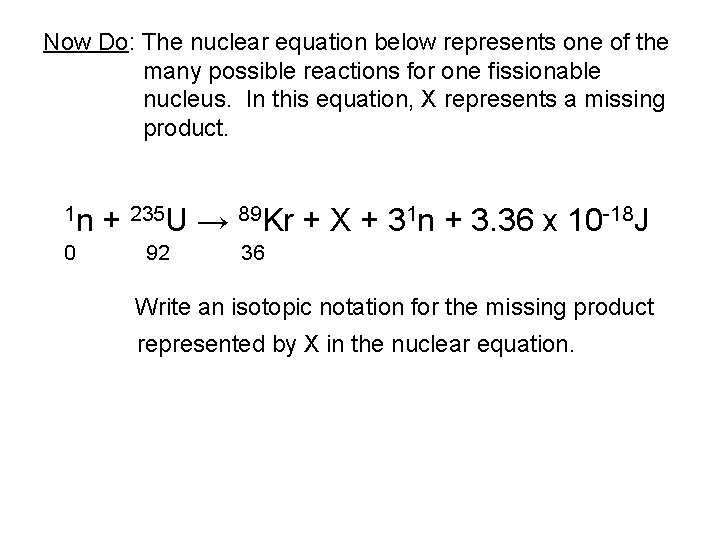
Now Do: The nuclear equation below represents one of the many possible reactions for one fissionable nucleus. In this equation, X represents a missing product. 1 n 0 + 235 U → 89 Kr + X + 31 n + 3. 36 x 10 -18 J 92 36 Write an isotopic notation for the missing product represented by X in the nuclear equation.

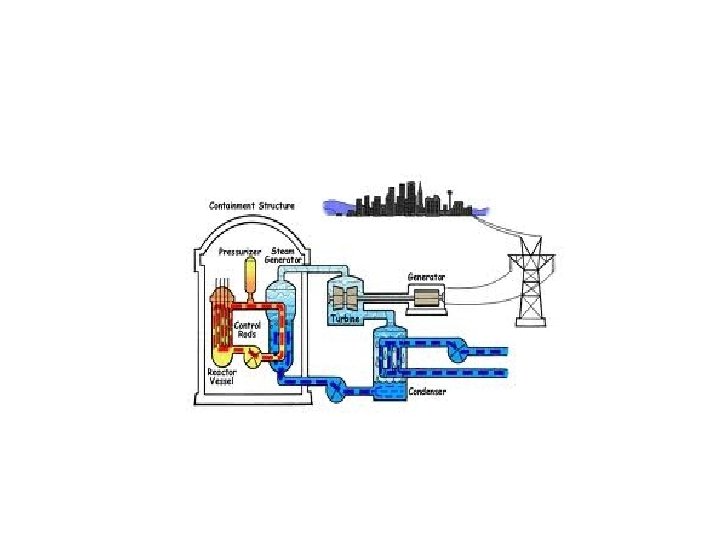
I Advantages A. Energy Source 1. nuclear fission heavy → 2 smaller nuclei + energy nucleus e. g. nuclear reactors/power plants


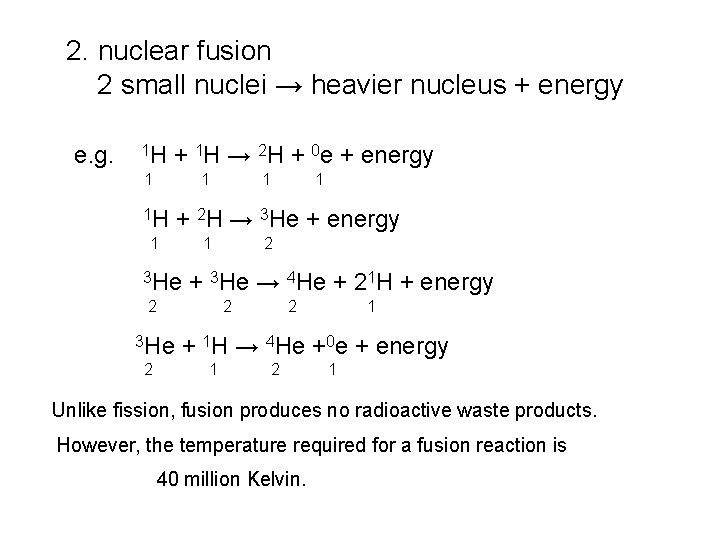
2. nuclear fusion 2 small nuclei → heavier nucleus + energy e. g. 1 H + 1 H → 2 H + 0 e + energy 1 1 1 H 1 3 He 2 + 3 He → 4 He + 21 H + energy 2 2 1 + 2 H → 3 He + energy 1 3 He 1 2 2 1 + 1 H → 4 He +0 e + energy 1 2 1 Unlike fission, fusion produces no radioactive waste products. However, the temperature required for a fusion reaction is 40 million Kelvin.

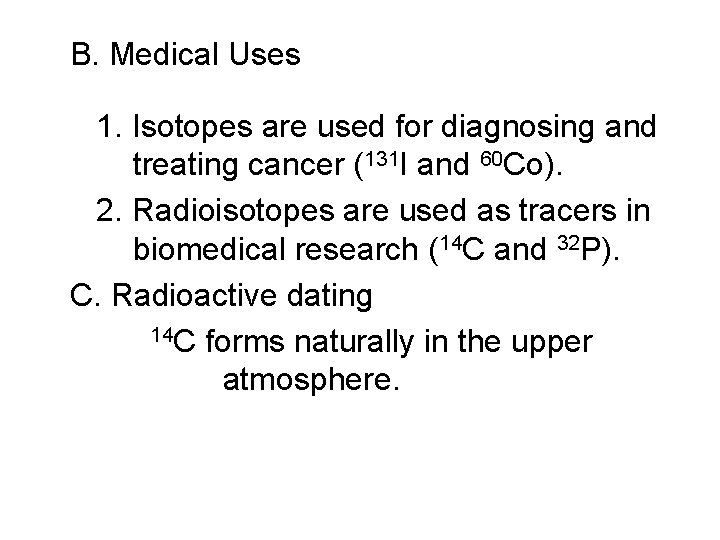
B. Medical Uses 1. Isotopes are used for diagnosing and treating cancer (131 I and 60 Co). 2. Radioisotopes are used as tracers in biomedical research (14 C and 32 P). C. Radioactive dating 14 C forms naturally in the upper atmosphere.

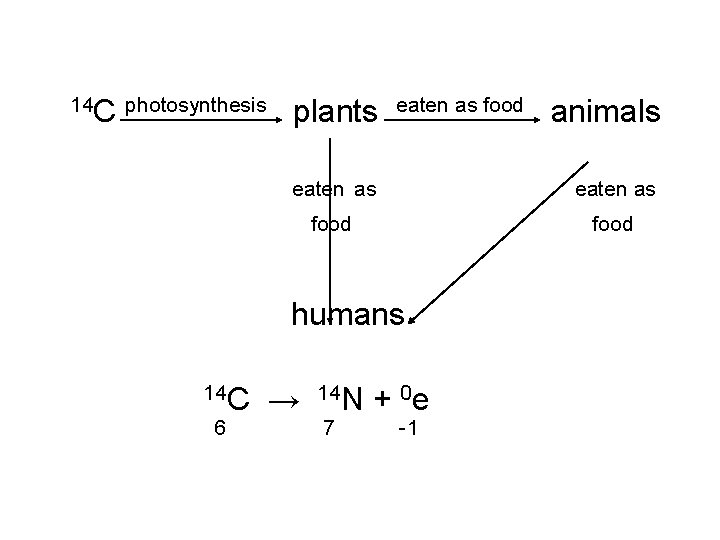
14 C photosynthesis plants eaten as food humans 14 C 6 animals → 14 N 7 + 0 e -1
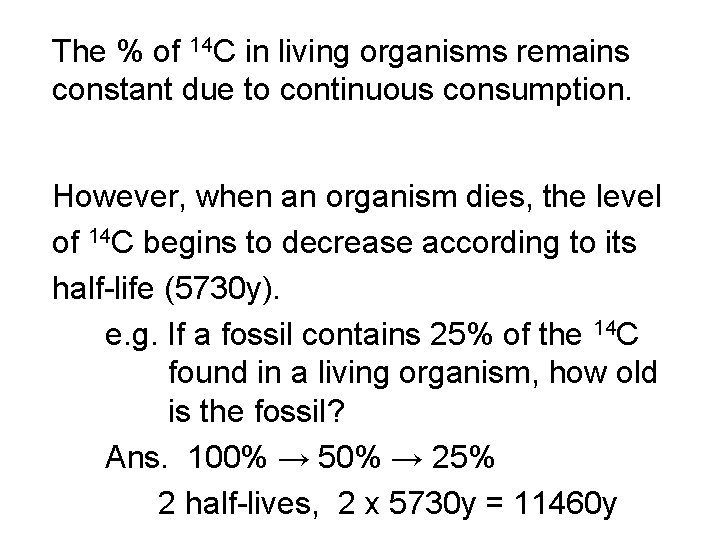
The % of 14 C in living organisms remains constant due to continuous consumption. However, when an organism dies, the level of 14 C begins to decrease according to its half-life (5730 y). e. g. If a fossil contains 25% of the 14 C found in a living organism, how old is the fossil? Ans. 100% → 50% → 25% 2 half-lives, 2 x 5730 y = 11460 y

D. Food Irradiation- increases shelf-life II Disadvantages A. Nuclear radiation (energy) is dangerous to living organismsit causes genetic damage and cancer. B. Nuclear waste- radioisotopes cannot be destroyed, and storage is a problem. C. Contamination of land water