Aim 45 What are the factors that affect


- Slides: 10

Aim # 45: What are the factors that affect solubility? H. W. # 45 Study pp. 514 – 521 (Sec. 11. 2 – 11. 3) p. A 33 (Appendix A 7. 5) Ans. ques. p. 548 # 93 p. 544 # 40, 43, 45, 47 p. 548 # 94 p. A (MC) # 1, 6 Do Now: Zumdahl 8 th ed. p. 531 #15 p. 536 # 98

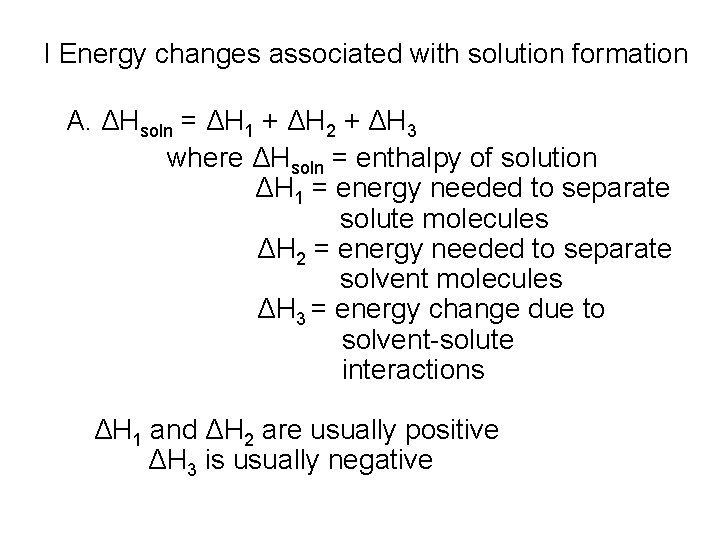
I Energy changes associated with solution formation A. ΔHsoln = ΔH 1 + ΔH 2 + ΔH 3 where ΔHsoln = enthalpy of solution ΔH 1 = energy needed to separate solute molecules ΔH 2 = energy needed to separate solvent molecules ΔH 3 = energy change due to solvent-solute interactions ΔH 1 and ΔH 2 are usually positive ΔH 3 is usually negative

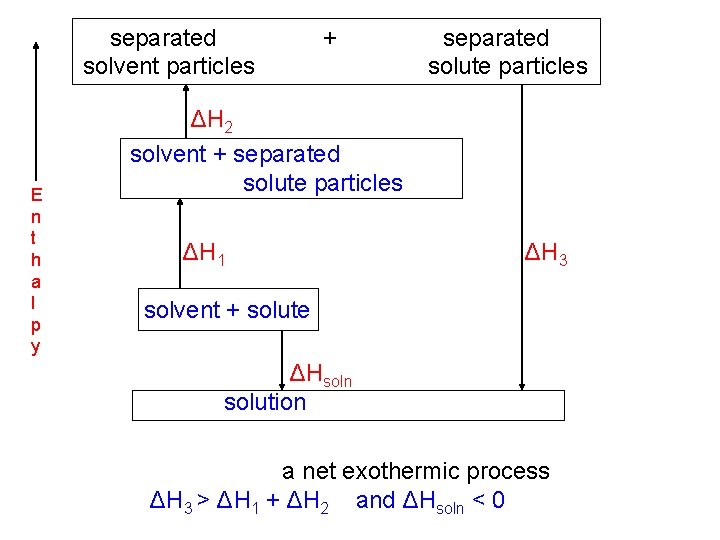
separated solvent particles E n t h a l p y + separated solute particles ΔH 2 solvent + separated solute particles ΔH 1 ΔH 3 solvent + solute ΔHsoln solution a net exothermic process ΔH 3 > ΔH 1 + ΔH 2 and ΔHsoln < 0

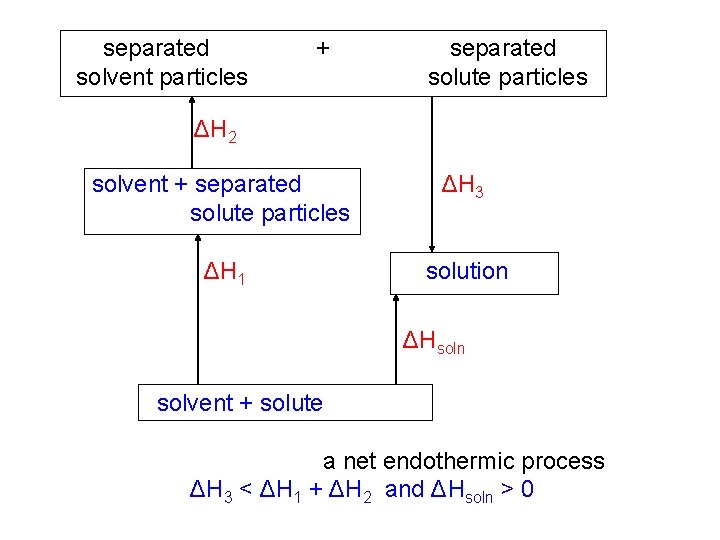
separated solvent particles + separated solute particles ΔH 2 solvent + separated solute particles ΔH 3 ΔH 1 solution ΔHsoln solvent + solute a net endothermic process ΔH 3 < ΔH 1 + ΔH 2 and ΔHsoln > 0

B. Factors affecting spontaneous solution formation 1. ΔH (change in enthalpy) A loss of energy favors spontaneous processes. 2. ΔS (change in entropy) Entropy is a measure of the randomness (or disorder) of a system. An increase in entropy favors spontaneous processes. If ΔS due to mixing is large enough, a solution will form spontaneously, even if ΔH is positive.

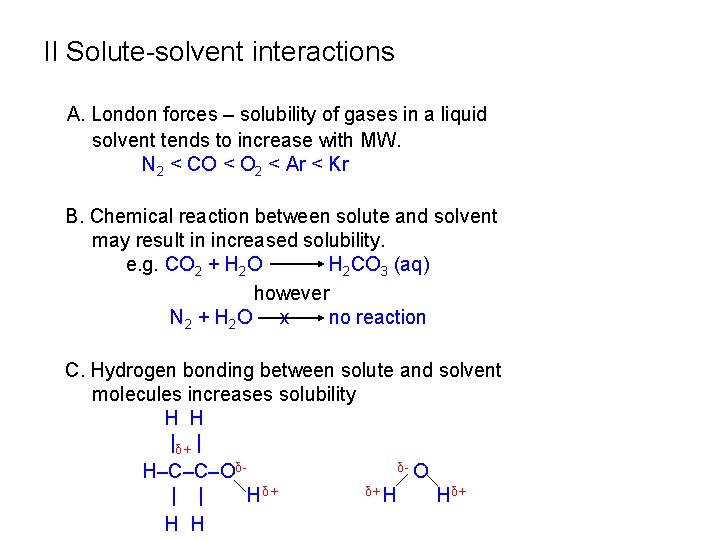
II Solute-solvent interactions A. London forces – solubility of gases in a liquid solvent tends to increase with MW. N 2 < CO < O 2 < Ar < Kr B. Chemical reaction between solute and solvent may result in increased solubility. e. g. CO 2 + H 2 O H 2 CO 3 (aq) however N 2 + H 2 O x no reaction C. Hydrogen bonding between solute and solvent molecules increases solubility H H |δ+ | δ- O H‒C‒C‒Oδδ+ H | | Hδ+ H H

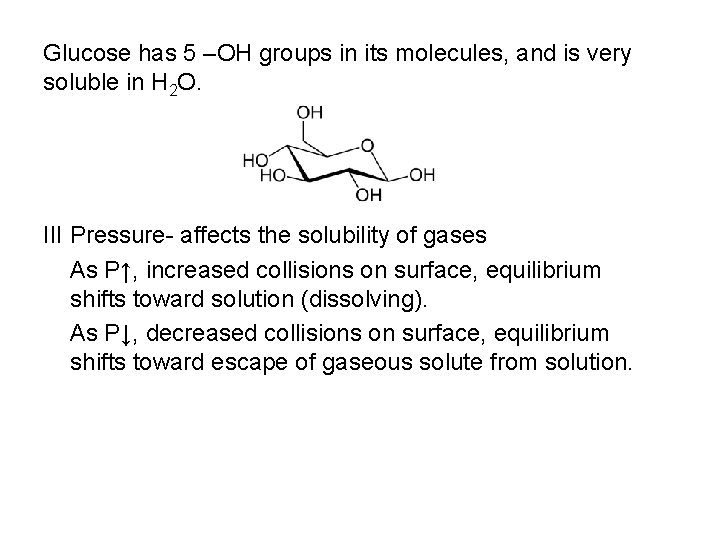
Glucose has 5 –OH groups in its molecules, and is very soluble in H 2 O. III Pressure- affects the solubility of gases As P↑, increased collisions on surface, equilibrium shifts toward solution (dissolving). As P↓, decreased collisions on surface, equilibrium shifts toward escape of gaseous solute from solution.

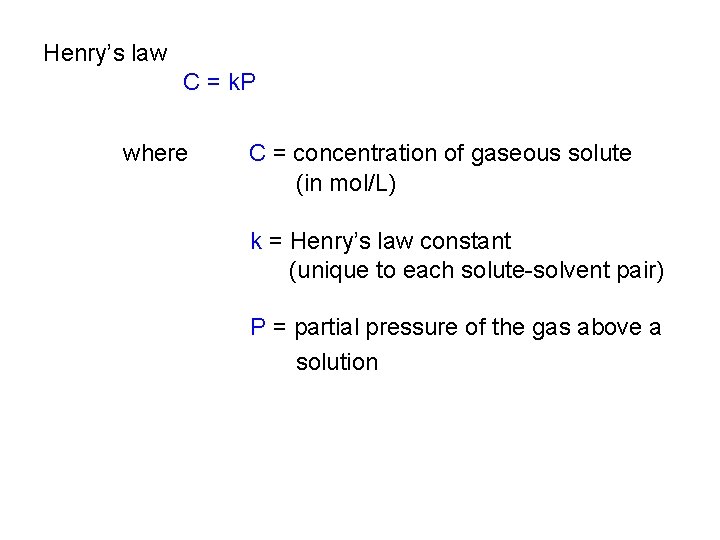
Henry’s law C = k. P where C = concentration of gaseous solute (in mol/L) k = Henry’s law constant (unique to each solute-solvent pair) P = partial pressure of the gas above a solution

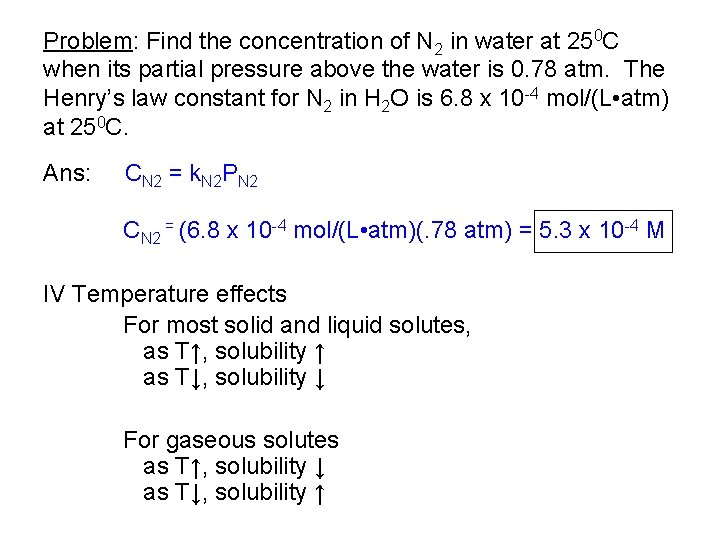
Problem: Find the concentration of N 2 in water at 250 C when its partial pressure above the water is 0. 78 atm. The Henry’s law constant for N 2 in H 2 O is 6. 8 x 10 -4 mol/(L • atm) at 250 C. Ans: CN 2 = k. N 2 PN 2 CN 2 = (6. 8 x 10 -4 mol/(L • atm)(. 78 atm) = 5. 3 x 10 -4 M IV Temperature effects For most solid and liquid solutes, as T↑, solubility ↑ as T↓, solubility ↓ For gaseous solutes as T↑, solubility ↓ as T↓, solubility ↑

Practice Problems Zumdahl (8 th ed. ) p. 532 # 39, 42, 49, 50, 48, 87