Aim 24 What factors determine whether a chemical








- Slides: 10

Aim # 24: What factors determine whether a chemical reaction will occur spontaneously? H. W. # 24 Study pp. 312 -315 Ans. ques. p. 315 #1, 4 p. 320 # 43, 44 Study class notes Complete the handout sheet

I A spontaneous physical or chemical reaction is one that can proceed of its own accord without outside (external) cause. e. g. 1) water freezes below 00 C 2) Ag. NO 3(aq) + Na. Cl(aq) → II Entropy- a measure of disorder, randomness, or lack of organization of a system. Higher entropy means greater disorder. The symbol of entropy is S. ΔS = Sfinal – Sinitial where Sfinal is the final entropy, after change has occurred.

Sinitial is the initial entropy before change has occurred. For a change to greater randomness, ΔS is positive. For a change to less randomness, ΔS is negative.

For each of the following, is ΔS positive or negative? Dissolving: Na. Cl(s) + H 2 O(l)→ Na+(aq) + Cl-(aq) Sublimation: CO 2(s) → CO 2(g) Decomposition: 2 KCl. O 3(s) → 2 KCl(s) + 3 O 2(g) Synthesis: 2 Mg(s) + O 2(g) → 2 Mg. O(s) Freezing: H 2 O(l) → H 2 O(s) All systems move naturally towards a state of greater randomness.

Which physical phase has the greatest amount of entropy? What effect should raising the temperature have on the entropy of a system?

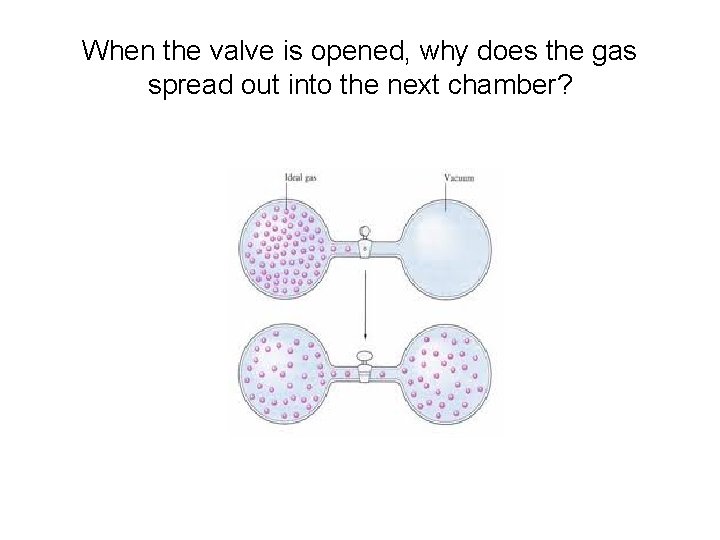
When the valve is opened, why does the gas spread out into the next chamber?

III Whether a chemical reaction occurs spontaneously depends upon the balance between two fundamental tendencies in nature: 1. toward a state of lower energy (lower enthalpy) and 2. toward greater randomness (higher entropy) IV At constant temperature and pressure, a system tends to undergo a reaction so that, in its final state, it has lower energy that in its initial state. ΔH is negative.

V At constant temperature, a system tends to undergo a reaction so that in its final state, it has a higher entropy (greater randomness) than in its initial state. ΔS is positive. VI In other words, -ΔH and +ΔS favor spontaneous change. +ΔH and –ΔS oppose spontaneous change.

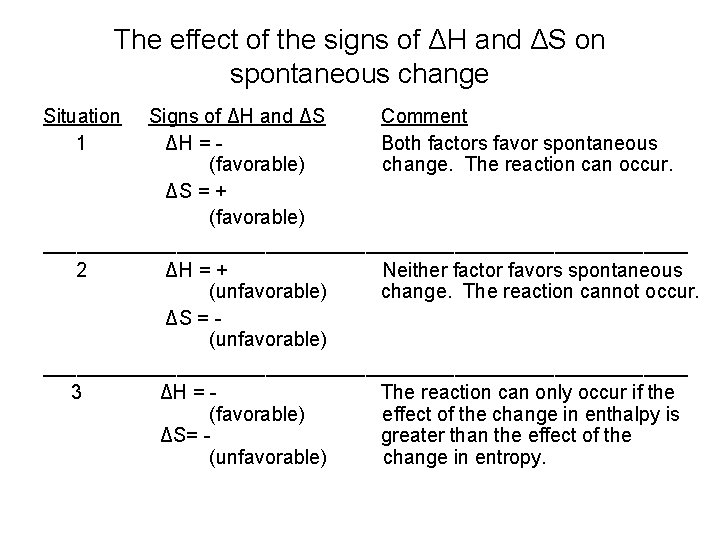
The effect of the signs of ΔH and ΔS on spontaneous change Situation 1 Signs of ΔH and ΔS Comment ΔH = Both factors favor spontaneous (favorable) change. The reaction can occur. ΔS = + (favorable) _____________________________ 2 ΔH = + Neither factor favors spontaneous (unfavorable) change. The reaction cannot occur. ΔS = (unfavorable) _____________________________ 3 ΔH = The reaction can only occur if the (favorable) effect of the change in enthalpy is ΔS= greater than the effect of the (unfavorable) change in entropy.

4 ΔH = + The reaction can only occur (unfavorable) if the effect of the change in entropy ΔS = + is greater than the effect of the (favorable) change in enthalpy. ____________________________ When ΔH and ΔS have the same sign, temperature determines whether change occurs spontaneously. The Gibbs free energy equationΔG = ΔH – TΔS If ΔG >0, spontaneous reaction occurs If ΔG <0, spontaneous reaction will not occur What happens when ΔG = 0?