AIM 2 How does temperature affect the vapor
















- Slides: 31

AIM # 2: How does temperature affect the vapor pressure of a liquid?


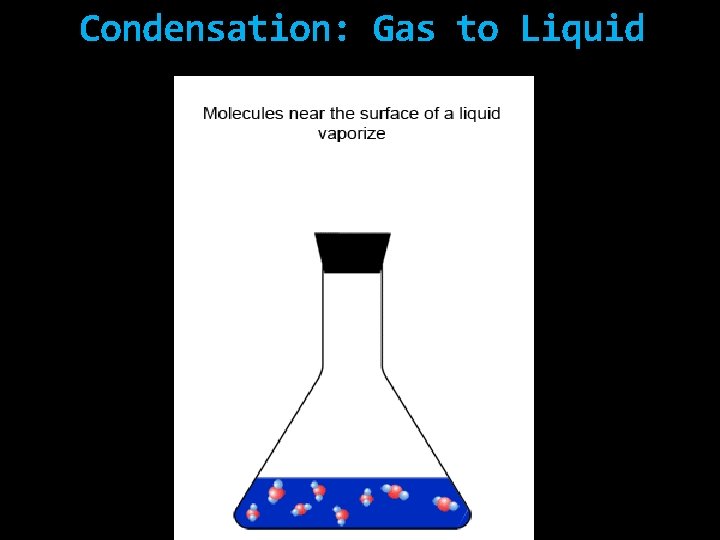
Vaporization: Liquid to Gas

Condensation: Gas to Liquid

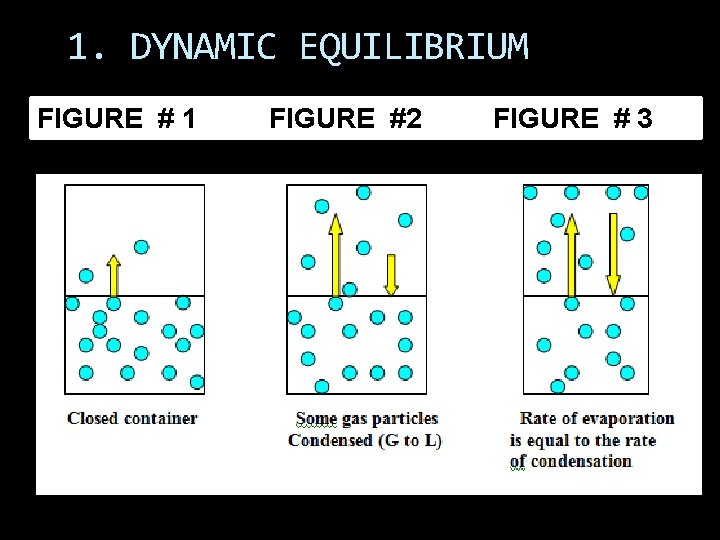
1. DYNAMIC EQUILIBRIUM FIGURE # 1 FIGURE #2 FIGURE # 3

Rate of Vaporization = Rate of Condensation Molecules are constantly changing phase: “Dynamic” The amount of liquid and vapor remains constant: “Equilibrium”

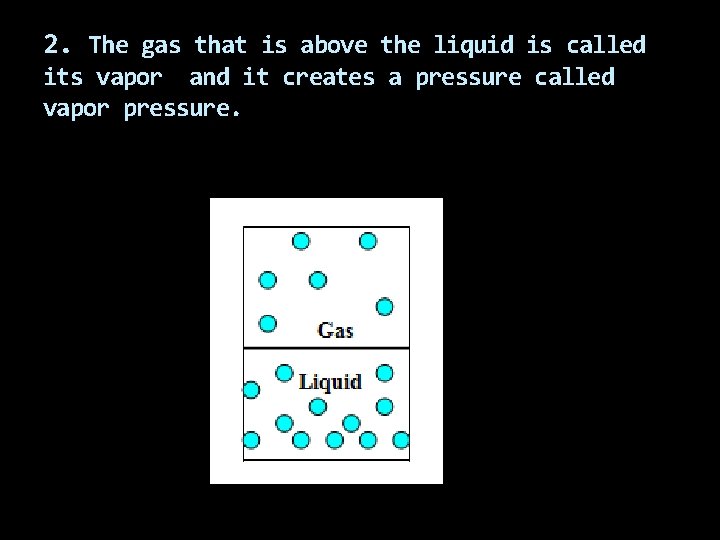
2. The gas that is above the liquid is called its vapor and it creates a pressure called vapor pressure.

3. The boiling Point of a Liquid The boiling point (bp) is the temperature at which the vapor pressure of the liquid is just equals the atmospheric pressure. VAPOR PRESSURE = ATMOSPHERIC PRESSURE

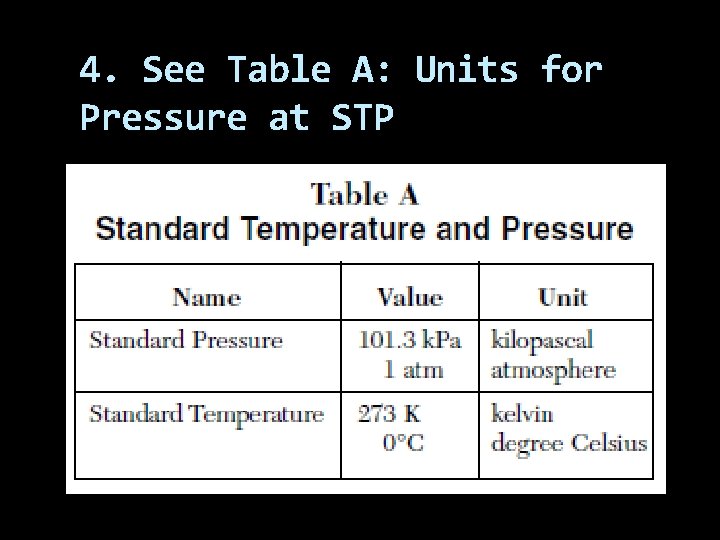
4. See Table A: Units for Pressure at STP



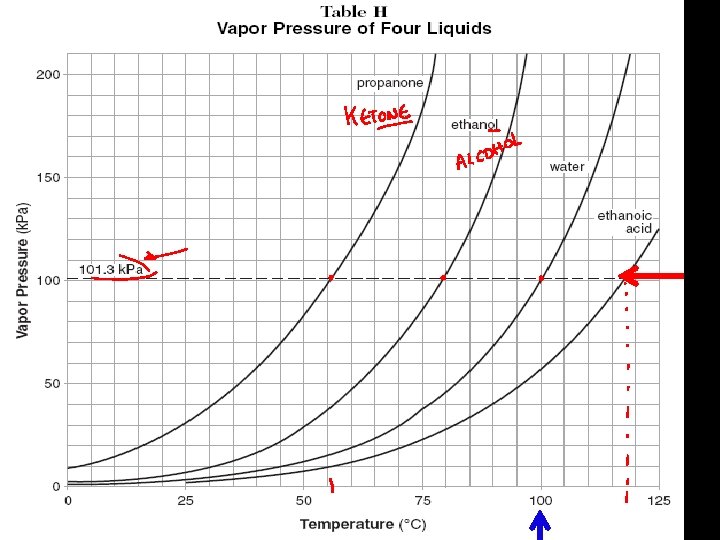
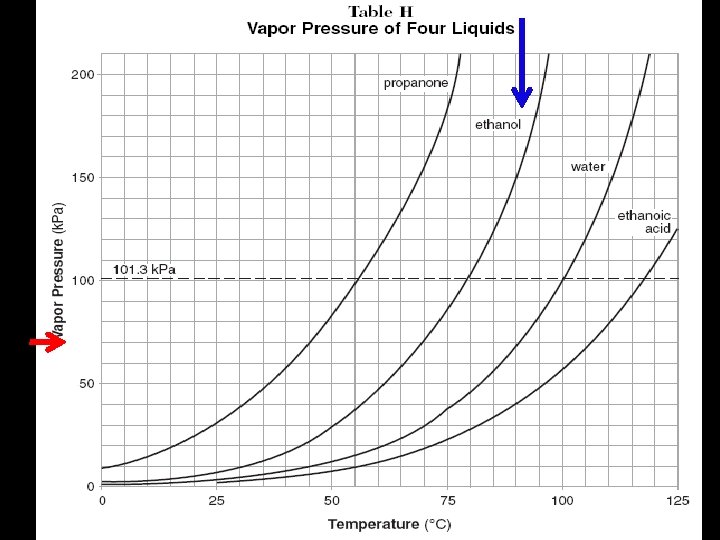
5. Factors That Affect Equilibrium Vapor Pressure (Table H) A. Temperature B. Intermolecular forces

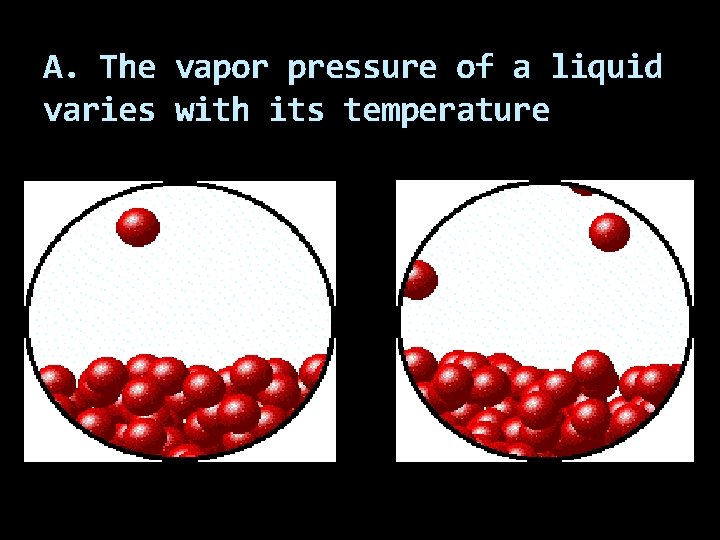
A. The vapor pressure of a liquid varies with its temperature

The vapor pressure is relatively high when the evaporation rate is high. As the temperature of a given liquid increases, so does its rate of evaporation and so does its vapor pressure

B. The stronger the forces between the particles the higher the boiling point.

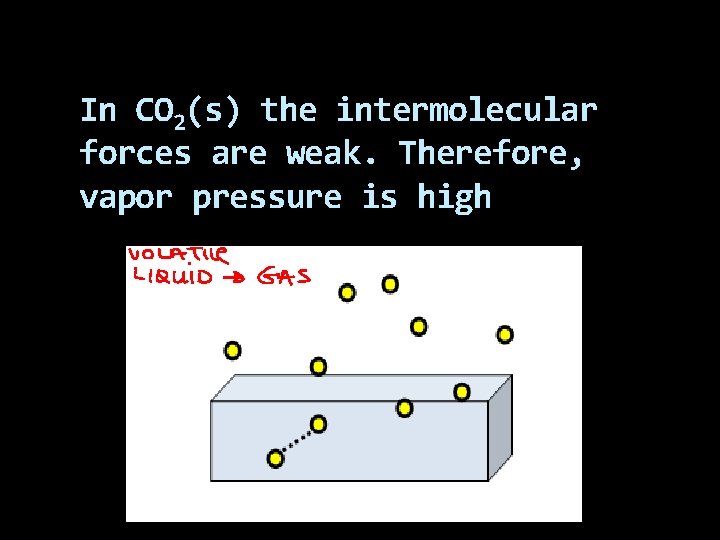
In CO 2(s) the intermolecular forces are weak. Therefore, vapor pressure is high

Nonburning Dollar Bill

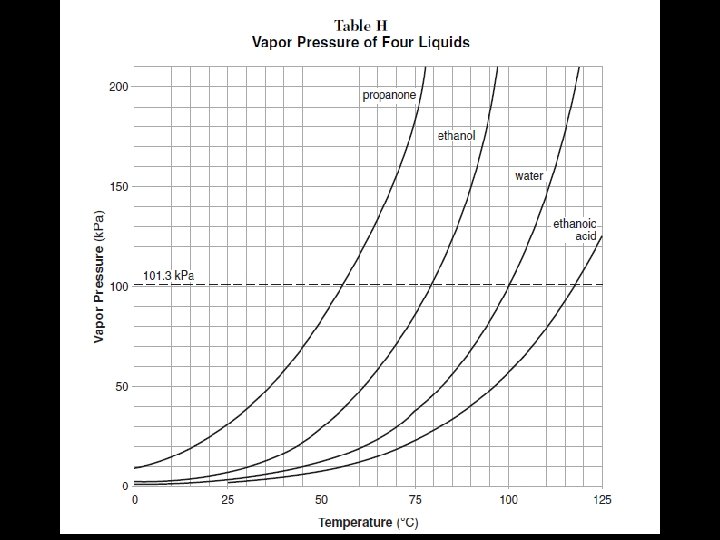
See Table H Intermolecular attraction are strongest in ethanoic acid (acetic acid), next strongest in water, third strongest in ethanol and weakest in propanone. Thus, we can use vapor pressures as indications of relative strengths of the attractive forces in liquids.

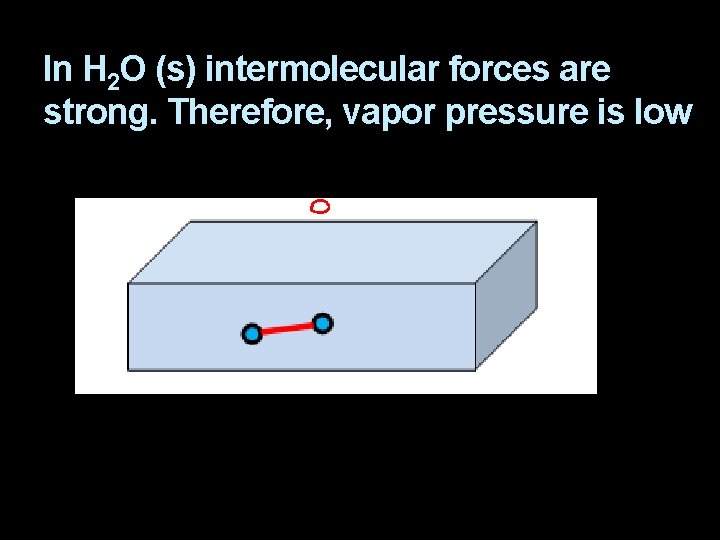
In H 2 O (s) intermolecular forces are strong. Therefore, vapor pressure is low


What property of ethyl alcohol makes it volatile? So ethanol is volatile, because it is a small and light molecule. The intermolecular forces are weak

Volatile (Table H) A liquid with a high vapor pressure at a given temperature is said to be volatile.

Volatile? Readily vaporizing at a relatively low temperature.

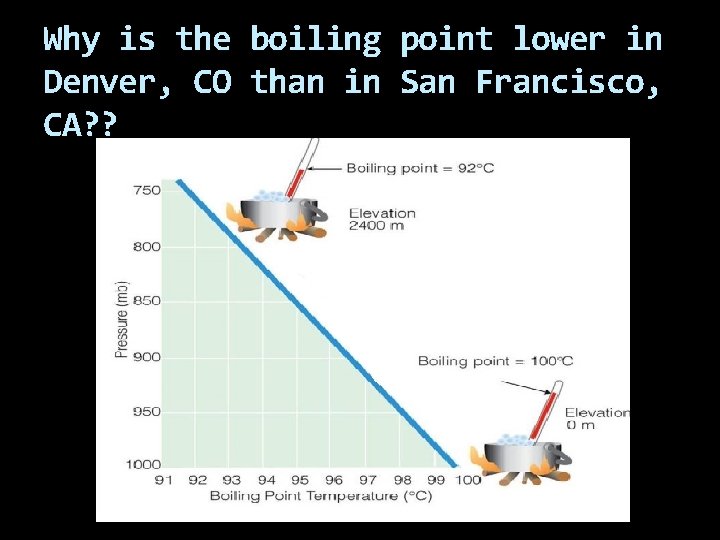
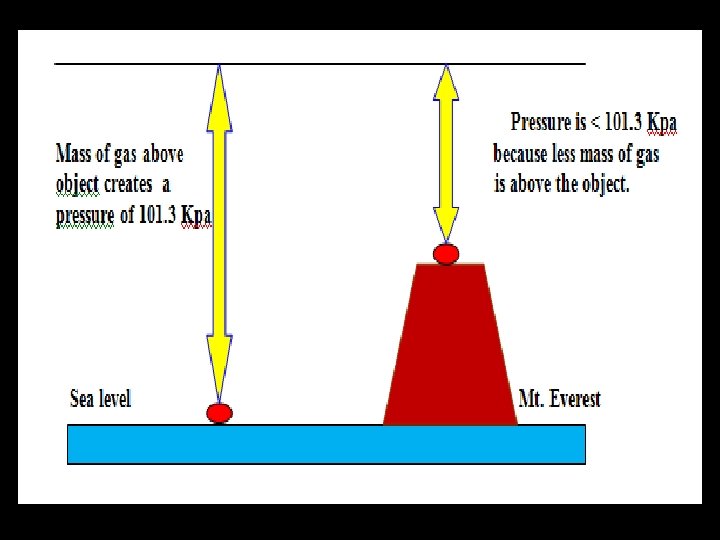
Why is the boiling point lower in Denver, CO than in San Francisco, CA? ?

Denver is at a higher altitude where the atmospheric pressure will be lower. Therefore the vapor pressure will be lower.

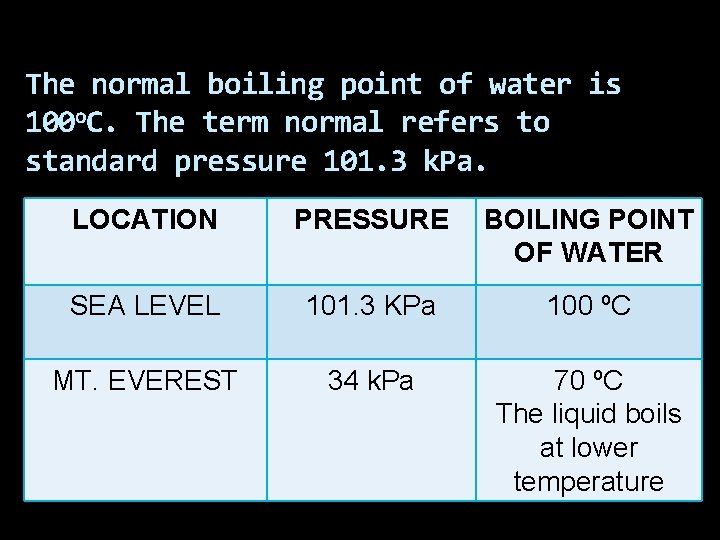
The normal boiling point of water is 100 o. C. The term normal refers to standard pressure 101. 3 k. Pa. LOCATION PRESSURE BOILING POINT OF WATER SEA LEVEL 101. 3 KPa 100 ºC MT. EVEREST 34 k. Pa 70 ºC The liquid boils at lower temperature


At high altitudes (up in the Mountains) the air pressure is less than at sea level. Thus, water will boil at a lower temperature (the vapor pressure needed to support a bubble is lower at high altitude). Therefore, cooking times are longer for things that need to be boiled (e. g. boiled eggs take longer to cook at high altitudes).

Changing the Boiling Point • Raise the external pressure (Use a pressure cooker) • Raises the vapor pressure needed. • Harder to make bubbles • Raises the boiling point. • Food cooks faster.

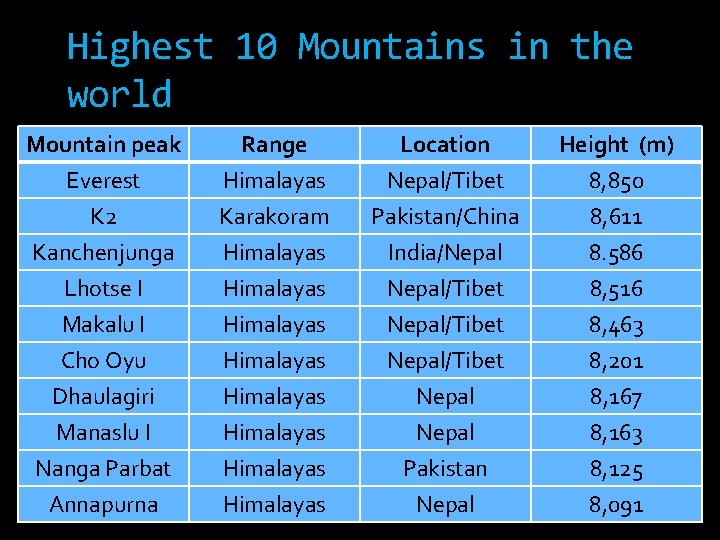
Highest 10 Mountains in the world Mountain peak Everest K 2 Kanchenjunga Range Himalayas Karakoram Himalayas Location Nepal/Tibet Pakistan/China India/Nepal Height (m) 8, 850 8, 611 8. 586 Lhotse I Makalu I Cho Oyu Dhaulagiri Manaslu I Nanga Parbat Annapurna Himalayas Himalayas Nepal/Tibet Nepal Pakistan Nepal 8, 516 8, 463 8, 201 8, 167 8, 163 8, 125 8, 091

END OF THE SHOW!