Aim 16 How can we use solubility products
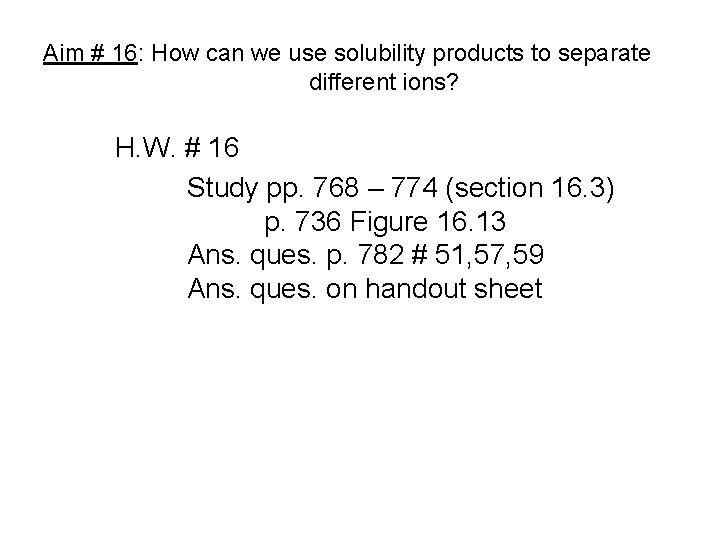
Aim # 16: How can we use solubility products to separate different ions? H. W. # 16 Study pp. 768 – 774 (section 16. 3) p. 736 Figure 16. 13 Ans. ques. p. 782 # 51, 57, 59 Ans. ques. on handout sheet
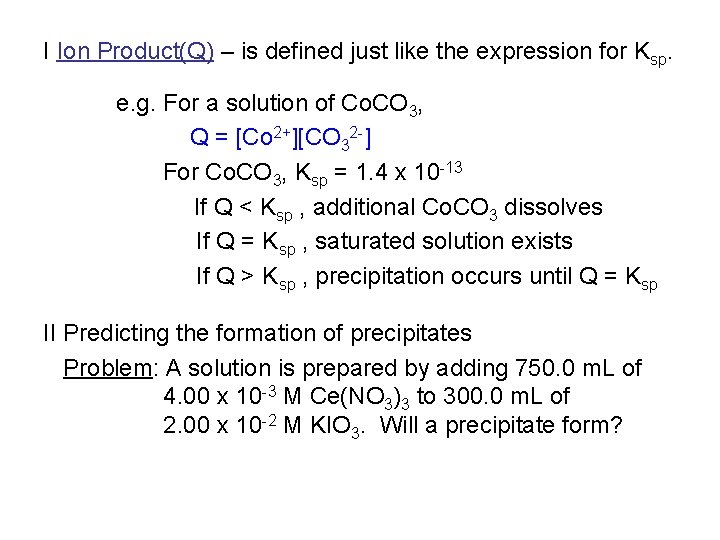
I Ion Product(Q) – is defined just like the expression for Ksp. e. g. For a solution of Co. CO 3, Q = [Co 2+][CO 32 -] For Co. CO 3, Ksp = 1. 4 x 10 -13 If Q < Ksp , additional Co. CO 3 dissolves If Q = Ksp , saturated solution exists If Q > Ksp , precipitation occurs until Q = Ksp II Predicting the formation of precipitates Problem: A solution is prepared by adding 750. 0 m. L of 4. 00 x 10 -3 M Ce(NO 3)3 to 300. 0 m. L of 2. 00 x 10 -2 M KIO 3. Will a precipitate form?
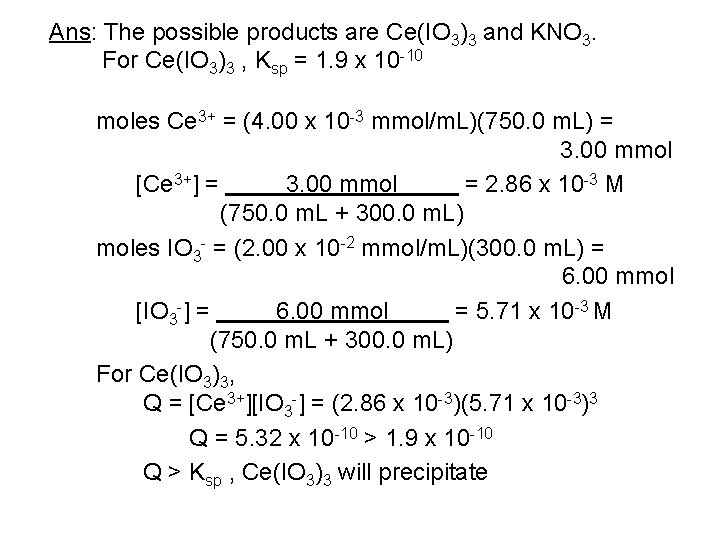
Ans: The possible products are Ce(IO 3)3 and KNO 3. For Ce(IO 3)3 , Ksp = 1. 9 x 10 -10 moles Ce 3+ = (4. 00 x 10 -3 mmol/m. L)(750. 0 m. L) = 3. 00 mmol [Ce 3+] = 3. 00 mmol = 2. 86 x 10 -3 M (750. 0 m. L + 300. 0 m. L) moles IO 3 - = (2. 00 x 10 -2 mmol/m. L)(300. 0 m. L) = 6. 00 mmol [IO 3 -] = 6. 00 mmol = 5. 71 x 10 -3 M (750. 0 m. L + 300. 0 m. L) For Ce(IO 3)3, Q = [Ce 3+][IO 3 -] = (2. 86 x 10 -3)(5. 71 x 10 -3)3 Q = 5. 32 x 10 -10 > 1. 9 x 10 -10 Q > Ksp , Ce(IO 3)3 will precipitate
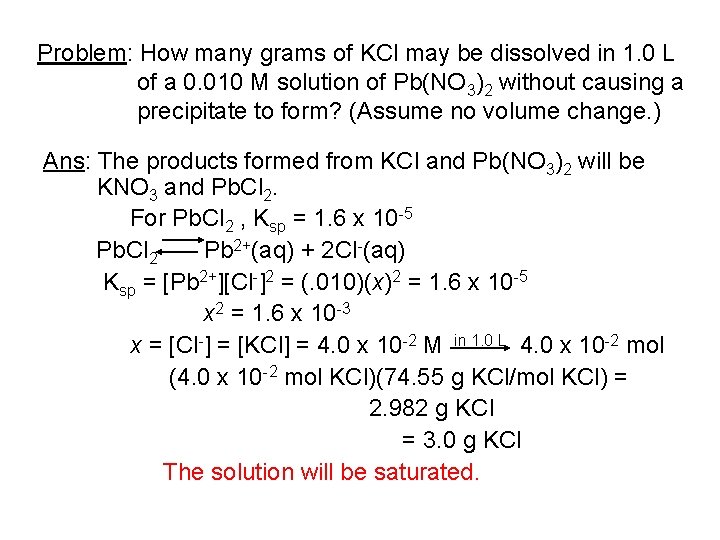
Problem: How many grams of KCl may be dissolved in 1. 0 L of a 0. 010 M solution of Pb(NO 3)2 without causing a precipitate to form? (Assume no volume change. ) Ans: The products formed from KCl and Pb(NO 3)2 will be KNO 3 and Pb. Cl 2. For Pb. Cl 2 , Ksp = 1. 6 x 10 -5 Pb. Cl 2 Pb 2+(aq) + 2 Cl-(aq) Ksp = [Pb 2+][Cl-]2 = (. 010)(x)2 = 1. 6 x 10 -5 x 2 = 1. 6 x 10 -3 x = [Cl-] = [KCl] = 4. 0 x 10 -2 M in 1. 0 L 4. 0 x 10 -2 mol (4. 0 x 10 -2 mol KCl)(74. 55 g KCl/mol KCl) = 2. 982 g KCl = 3. 0 g KCl The solution will be saturated.
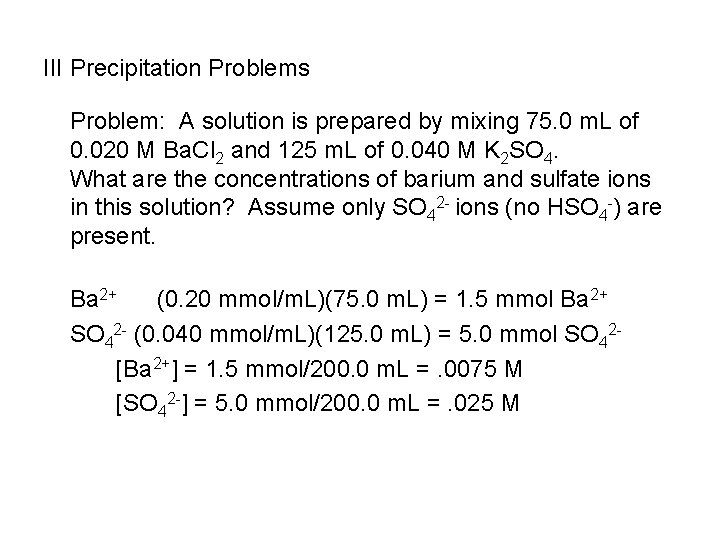
III Precipitation Problems Problem: A solution is prepared by mixing 75. 0 m. L of 0. 020 M Ba. Cl 2 and 125 m. L of 0. 040 M K 2 SO 4. What are the concentrations of barium and sulfate ions in this solution? Assume only SO 42 - ions (no HSO 4 -) are present. Ba 2+ (0. 20 mmol/m. L)(75. 0 m. L) = 1. 5 mmol Ba 2+ SO 42 - (0. 040 mmol/m. L)(125. 0 m. L) = 5. 0 mmol SO 42[Ba 2+] = 1. 5 mmol/200. 0 m. L =. 0075 M [SO 42 -] = 5. 0 mmol/200. 0 m. L =. 025 M
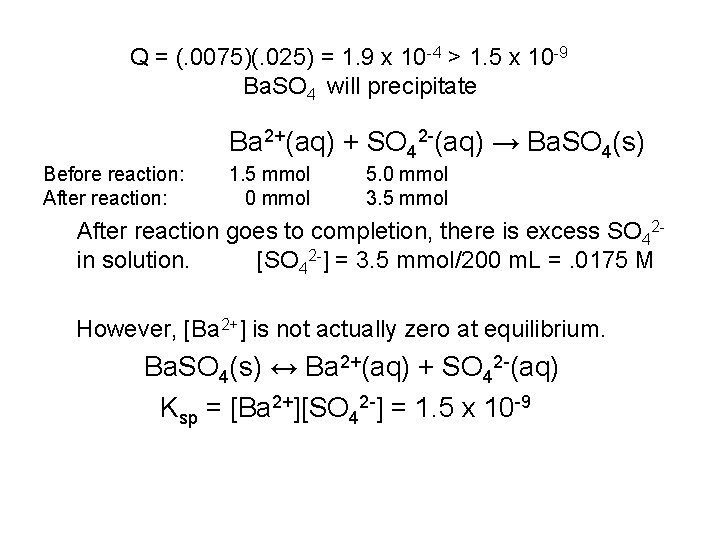
Q = (. 0075)(. 025) = 1. 9 x 10 -4 > 1. 5 x 10 -9 Ba. SO 4 will precipitate Ba 2+(aq) + SO 42 -(aq) → Ba. SO 4(s) Before reaction: After reaction: 1. 5 mmol 0 mmol 5. 0 mmol 3. 5 mmol After reaction goes to completion, there is excess SO 42 in solution. [SO 42 -] = 3. 5 mmol/200 m. L =. 0175 M However, [Ba 2+] is not actually zero at equilibrium. Ba. SO 4(s) ↔ Ba 2+(aq) + SO 42 -(aq) Ksp = [Ba 2+][SO 42 -] = 1. 5 x 10 -9
![Initial conc. (mol/L) Equilibrium conc. (mol/L) [Ba 2+]0 = 0 [Ba 2+] = x Initial conc. (mol/L) Equilibrium conc. (mol/L) [Ba 2+]0 = 0 [Ba 2+] = x](http://slidetodoc.com/presentation_image/f956148c24cc5e63cbfbb6536ed1d747/image-7.jpg)
Initial conc. (mol/L) Equilibrium conc. (mol/L) [Ba 2+]0 = 0 [Ba 2+] = x [SO 42 -]0 =. 0175 [SO 42 -] =. 0175 + x x mols Ba. SO 4 dissolved Ksp = 1. 5 x 10 -9 = [Ba 2+][SO 42 -] = (x)(. 0175 + x) Ksp ≈ (x)(. 0175) x = [Ba 2+] = 8. 6 x 10 -8 M [SO 42 -] =. 018 M

IV Selective precipitation of ions Problem: A solution contains 1. 0 x 10 -4 M Cu+ and 2. 0 x 103 M Pb 2+. If a source of I- is gradually added to this solution, will Pb. I 2 (Ksp = 1. 4 x 10 -8) or Cu. I (Ksp = 5. 3 x 10 -12) precipitate first ? What concentration of I- is necessary to begin precipitation of each salt? Ans: For Pb. I 2, the Ksp expression is 1. 4 x 10 -8 = Ksp = [Pb 2+][I-]2 1. 4 x 10 -8 = (2. 0 x 10 -3)[I-]2 [I-] = √(1. 4 x 10 -8)/(2. 0 x 10 -3) = 2. 6 x 10 -3 M Any I- in excess of this conc. will cause Pb. I 2 to form.
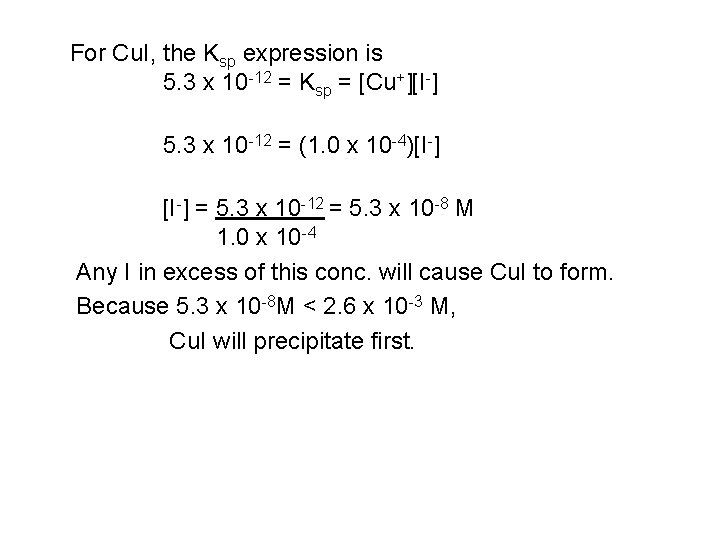
For Cu. I, the Ksp expression is 5. 3 x 10 -12 = Ksp = [Cu+][I-] 5. 3 x 10 -12 = (1. 0 x 10 -4)[I-] = 5. 3 x 10 -12 = 5. 3 x 10 -8 M 1. 0 x 10 -4 Any I in excess of this conc. will cause Cu. I to form. Because 5. 3 x 10 -8 M < 2. 6 x 10 -3 M, Cu. I will precipitate first.

Practice Problems Zumdahl (8 th ed. ) p. 767 # 51 p. 769 # 85 p. 767 # 54, 58
- Slides: 10